Chapter: Modern Medical Toxicology: Cardiovascular Poisons: Diurets, Antihypertensives and Antiarrhythmics
Calcium Channel Blockers - Sympatholytic Drug Cardiovascular Poison
Calcium Channel Blockers
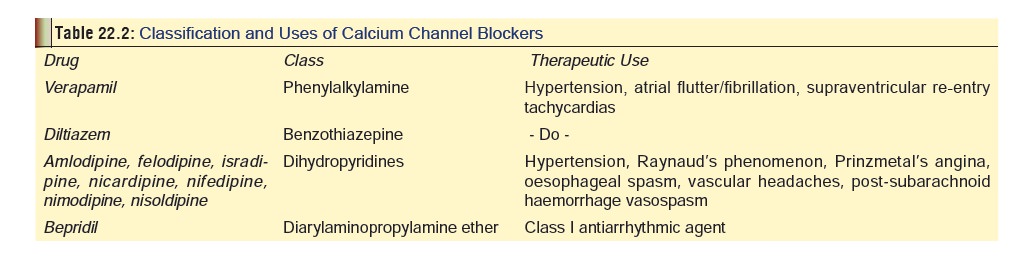
Calcium channel blockers (calcium antagonists; slow channel blockers) block the influx of calcium into various cells, primarily vascular, cardiac, and smooth muscle tissue. They are used primarily for treatment of supraventricular tachy-cardia, angina, and hypertension. Examples include amlodi-pine, bepridil, diltiazem, felodipine, isradipine, lacidipine, mibefradil, nicardipine, nifedipine, nimodipine, nisoldipine, perhexiline, verapamil, etc. (See Table 22.2 for classification).Newer agents include aranidipine, lercanidipine, nilvadipine,nitrendipine, tiapamil.
Mode of Action
·
Calcium antagonists selectively
inhibit membrane transport of calcium during the slow inward
excitation-contraction coupling phase in cardiac and vascular smooth muscle.
Intracellular calcium ion outflow may also be speeded through stimulation of
ATP dependant Ca and Na-K pumps
·
All calcium channel blockers (CCBs)
act by antagonising L-type voltage-sensitive slow calcium channels, except
mibefradil which blocks T channels. L-channel blockade impairs calcium influx
into cardiac and smooth muscle cells, resulting in decreased force of
myocardial contrac-tion, negative inotropy, inhibition of SA and AV nodes, and
peripheral arteriolar vasodilatation.
·
Calcium antagonists selectively
inhibit membrane transport of calcium during the slow inward
excitation-contraction coupling phase in smooth muscle leading to coronary and
peripheral vasodilation. In general, they have a negative inotropic
(contractility) effect on the myocardium not usually manifested with
therapeutic doses due to compen-sation of the sympathetic nervous system.
·
Verapamil has the most powerful
myocardial depressant effect, while diltiazem has much less effect, and
nifedipine is a weak myocardial depressant but exerts very significant effects
on peripheral vascular smooth muscle. Therefore verapamil is the most potent at
lowering heart rate, cardiac output, and blood pressure, while nifedipine
produces maximum decrease in systemic vascular resistance.
Toxicokinetics
·
All CCBs are absorbed well orally
and are highly protein-bound. Verapamil, diltiazem, and nifedipine undergo
extensive hepatic metabolism. Volumes of distribution are large for the former
two (about 5.5 L/kg), but much smaller for nifedipine (0.8 L/ kg). Amlodipine
differs from the other members of its class (dihydropyridines) in that it has a
very long plasma half-life (35 to 45 hours), and prolonged duration of action.
Adverse Effects
·
Dizziness, flushing, headache,
oedema, palpitations, hypoten-sion, GI upsets. Gingival hyperplasia has been
noted with amlodipine.
Drug Interactions
·
Severe bradycardia, conduction
blocks and hypotension have been reported in patients taking calcium
antagonists and beta blockers at therapeutic doses and in overdose.
·
Mibefradil appears to interfere with
the body’s metabolism of lovastatin and simvastatin (and also possibly
atorvastatin and cerivastatin), and increase the risk of muscle injury.
Fluvastatin and pravastatin do not have similar metabolism and mibefradil would
not be expected to increase the risk of muscle injury with these agents.
·
QT interval prolongation has been
associated with concur- rent administration of cisapride and diltiazem.
·
Complete AV block and QTc
prolongation were reported following concurrent administration of high-dose
verapamil and erythromycin, which may be due to a pharmacokinetic interaction
between the two drugs as a result of both drugs
Clinical (Toxic) Features
·
Early manifestations may be mild such as dizziness and
lethargy. GI manifestations such as vomiting and diar-rhoea are relatively
uncommon.
·
Bradycardia, hypotension, A-V conduction anomalies,
idioventricular rhythms, complete heart-block. Heart rates below 60 beats/min
with accompanying hypo-tension at presentation are common. It is important to
remember that patients who are asymptomatic on admission may subsequently
suddenly deteriorate into profound cardiogenic shock.
·
Nifedipine and amlodipine lack the effects of other
struc-tural classes of CCBs on AV nodal conduction. Therefore, these agents are
more likely to result in reflex tachycardia secondary to diminished perfusion;
bradycardia is twice as likely with verapamil and diltiazem.
·
AV block, especially greater than first degree, is
predomi-nately a finding with verapamil. ECG manifestations following verapamil
intoxication include heart block, first, second and third degree AV block,
junctional rhythm, QT interval prolongation, moderate S-T segment depression,
low amplitude T-waves, prominent U-waves, and atrial fibrillation. Cardiac
disturbances commonly persist for 9 to 48 hours, but have been reported to last
as long as 7 days.
·
Symptoms may be delayed and of prolonged duration following
ingestion of sustained-release dosage forms. Gastric concretions from
sustained-release dosage forms have been found at autopsy. Gastroscopy may be
required for confirmation if suspected in the living patient, since these
masses have not been apparent on abdominal films.
·
In severe poisoning, altered mental status, convulsions,
stroke, renal failure, non-cardiogenic pulmonary oedema, and coma can occur.
Noncardiogenic pulmonary oedema has been reported following diltiazem,
verapamil, and amlodipine overdose.
·
Hyperglycaemia has been reported in several cases, probably
because normal calcium influx is impaired by CCBs which affects insulin release
from beta cells in the pancreas.
·
Acute renal failure has been reported, usually in patients
who develop prolonged hypotension and/or rhabdomy-olysis after severe
poisoning.
·
Profound hypocalcaemia (with tetany) can occur.
·
CNS depression, secondary to haemodynamic insta-bility
occurs following significant overdose. Effects may include drowsiness,
confusion, and coma. Cerebral infarction has been reported. Seizure activity
may result from acidosis, anoxia, or an existing predisposition.
·
Toxicity is likely to be more severe in elderly patients,
young children, patients with underlying CVS disease, and co-ingestions with beta-adrenergic
antagonists, digoxin, or other drugs with cardiovascular activity.
Sustained-release preparations are associated with delayed presentation
(sometimes upto 15 hours), and much longer duration of toxicity.
Treatment
·
Intravenous access; continuous ECG monitoring. Monitor
haemodynamic status closely including heart rate, blood pressure, continuous
cardiac monitoring and serial ECG, and urinary output. Obtain 12-lead ECG
demonstrating the rhythm and intervals; repeat every 2 hours for the first 8
hours, and then at longer intervals subsequently.
·
Monitor electrolytes, renal function tests and glucose;
monitor respiratory function with arterial blood gases.
·
CCBs are generally radiolucent. Concretions of
sustained-release preparations may be apparent on abdominal radiographs.
·
Airway protection; oxygenation.
·
GI decontamination: stomach wash and activated char-coal.
For overdoses involving sustained-release prepara-tions, whole bowel irrigation
with polyethylene glycol is said to be beneficial. Repeat charcoal following
whole bowel irrigation since the PEG/electrolyte solution may desorb drug from
charcoal. If continued absorption is suspected in a symptomatic patient after
these proce-dures, consider abdominal X-ray (if brand is radiopaque), ultrasound,
or gastroscopy.
·
Patients who show the following signs of toxicity, (or any
patient with a history of ingestion of sustained release dosage forms) should
be admitted to a monitored setting for at least 24 hours of observation and
treatment, inde-pendent of the dose ingested:
o
CVS—Hypotension or bradycardia (or tachycardia with
nifedipine); heart block; A-V dissociation; asystole; congestive heart failure
o
RS—Pulmonary oedema
o
GI—Nausea or vomiting
o
CNS—Seizures; altered mental status
·
Bradycardia usually responds to atropine, the efficacy of
which may be enhanced by initial treatment with calcium. Dosage recommended is
0.5 to 1 mg IV every 2 to 3 minutes to a maximum of 3 mg. In children: 0.02
mg/kg.
·
Calcium therapy: 10% calcium chloride, 10 to 20 ml, IV, or
calcium gluconate, 30 to 60 ml, IV, and repeated every 15 to 20 minutes, upto 4
doses. Alternatively, calcium can be administered as an infusion: 0.2 to 0.4
ml/kg/hr of 10% Calcium chloride, or 0.6 to 1.2 ml/kg/hr of 10% Calcium
gluconate. While calcium therapy is beneficial in CCB overdose, serum Calcium
should be monitored to prevent hypercalcaemia. However, some degree of
hypercalcaemia may be necessary before severely intoxi-cated patients respond
to aggressive calcium therapy. Hence, some authors advocate administering 1
gram of calcium salts every 2 to 3 minutes until conduction block is reversed
or clinical evidence of hypercalcaemia develops. Use of calcium chloride may
aggravate existing acidosis. Calcium therapy is contraindicated in ingestions
involving digoxin.
·
Hypotension secondary to reduced systemic resistance and
lowered cardiac output may require both fluid replacement, Trendelenburg
positioning and vasocon-striction with noradrenaline or high dose dopamine.
Calcium may also help, especially when depressed cardiac contractility is
contributory. Glucagon may improve perfusion pressure by stimulating cardiac
output. Pacing may be required. Catecholamines and sympatho-mimetics such as
adrenaline, noradrenaline, dopamine, isoproterenol, and dobutamine have been
used with varying degrees of success in CCB poisoning.
·
Glucagon has been reported to be beneficial by several
investigators. It exerts chronotropic and inotropic effects and can help
reverse hypotension, but may not improve heart rate. Dose: 2 to 5 mg IV over 1
minute, followed by 4 to 10 mg over 5 minute (adults); 50 mcg/kg (children).
Because of the short half-life of glucagon, a maintenance infusion is
subsequently necessary at the “response dose”, i.e. the initial effective dose.
Continuous infusion of up to 5 mg/hr has been used with benefit.
·
Conduction deficits and bradyarrhythmias do not need
specific treatment if they are not felt to be contributing to continuing
hypotension. Antidotal therapy should include calcium (as the chloride) and/or
atropine initially, followed by isoproterenol and/or pacing for resistant or
nonresponsive cases.
·
Inamrinone, a non-catecholamine inotropic agent has also
been used in CCB poisoning with encouraging results. It is usually combined
with glucagon or some other inotropic agent such as isoproterenol. Dose : 1
mg/kg IV over 2 minutes, followed by infusion of 5 to 20 mcg/kg/min.
·
Other drugs which are being tried include 4-aminopyri-dine,
and insulin-plus-glucose. The latter can be admin-istered as bolus doses, 10 IU
and 25 grams respectively, with the subsequent administration of insulin
infusion, the dose ranging from 0.1 IU/kg/hr to 1.0 IU/kg/hr, and ![]() dextrose (50% w/v) infusion, the
dose ranging from 5 gm/hr to 15 gm/hr, via a central venous catheter. Insulin
infusions, with or without dextrose given concurrently, have been administered
with beneficial effects to several haemodynamically unstable patients following
calcium antagonist intoxication who were refractory to conven-tional therapy.
dextrose (50% w/v) infusion, the
dose ranging from 5 gm/hr to 15 gm/hr, via a central venous catheter. Insulin
infusions, with or without dextrose given concurrently, have been administered
with beneficial effects to several haemodynamically unstable patients following
calcium antagonist intoxication who were refractory to conven-tional therapy.
·
Patients not responding to pharmacologic therapy may require
transthoracic or intravenous cardiac pacing. Newer methods include intra-aortic
balloon counterpul-sation and emergent cardiopulmonary bypass.
·
Seizures should be treated with diazepam initially,
progressing to phenobarbitone for nonresponsive cases. Correction of underlying
metabolic acidosis, hypoxia, and hypotension should also be pursued.
·
In general, the large volumes of distribution and high
protein binding of all calcium channel blocking agents would suggest haemodialysis
or haemoperfusion would have limited usefulness in removal of significant
quanti-ties of these drugs.
Related Topics