Chapter: 9th Science : Applied Chemistry
Applications of Radiochemistry
Radiochemistry
You have studied in
previous chapters that elements can exist in nature as their isotopes. Isotopes
are atoms with the same number of protons and electrons, but a different number
of neutrons. Some isotopes are stable and stay forever. These are the elements
that we see around us and find in nature. However, some isotopes are unstable
and they undergo disintegration by losing their energy in the form of
radiation. As we studied earlier, every element tries to attain stability by
sharing, losing or gaining electrons (octet rule). Thus the unstable isotopes
of elements lose their energy in the form of radiation to become stable.
This phenomenon is
called radioactive decay. The isotope which undergoes radioactive
decay is called radioactive isotope or radioisotope. This
property of isotopes is known as radioactivity.
Radiochemistry is the study of
chemistry of radioactive and non-radioactive isotopes. It includes both
natural and artificial isotopes. Radiochemistry mainly deals with application
of radioisotopes to study the nature of chemical reactions of non-radioactive
isotopes of elements and applications of radioisotopes to various fields.
Applications of Radiochemistry
Radioisotopes can easily
be detected and estimated quantitatively. So they are used in radiochemistry
for various applications. Radiochemistry mainly deals with study of chemical
reactions of non-radioactive isotopes using radioisotopes. In addition to that
it could find applications in medical field and environmental management also.
Let us list important applications of radioisotopes.
Radiocarbon dating: It is a method by which
the age of fossil wood or animal is determined using C-14 isotope.
Study of chemical
reactions: The nature of some of the chemical reactions can be studied
by mixing a radioisotope with non-radioactive isotope of the reactants. The
radioisotope used for this purpose is called radiotracer. For example,
by photosynthesis plants synthesize carbohydrate from carbon dioxide and water
as shown in the following reaction.
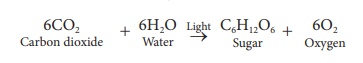
Here a question arises
that whether the oxygen evolved in this process comes from CO2 or H2O.
By using radioisotope O-18 as tracer, it was found that the evolved oxygen
comes from H2O.
Diagnosis: Radioisotopes are found
very useful to diagnose and understanded many diseases.
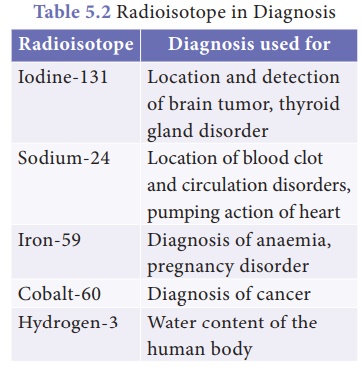
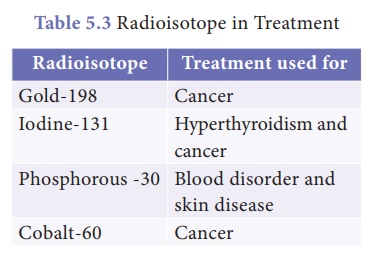
Radiotherapy: Radioactive isotopes are
used in the treatment of many diseases. This kind treatment is called
radiotherapy.
Related Topics