Chapter: Modern Medical Toxicology: Analytical Toxicology: Analytical Instrumentation
Applications of High Performance Liquid Chromatography (HPLC)
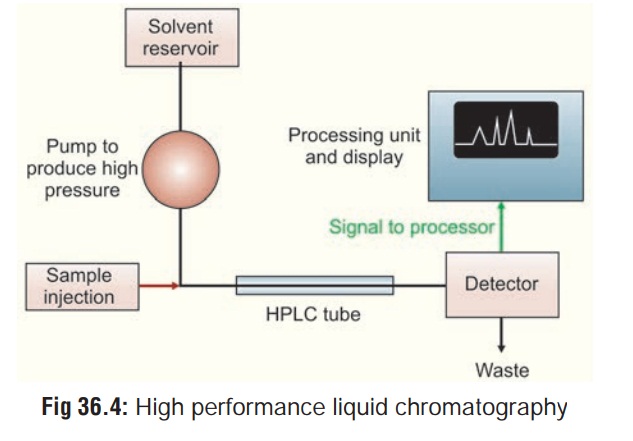
High Performance Liquid Chromatography (HPLC)
This is similar to GC, except that
it is not restricted to volatile compounds. A high pressure (1000 to 6000 pound
per square inch) pump facilitates movement of the specimen through the columns
packed with chromatographic adsorbents e.g. silica gel and alumina. The
effluent stream passes through a detector, usually an ultraviolet
spectrophotometer, and the appearance of a drug in the solvent is signalled by
a recorder peak in the same way as in GC (Fig
36.4). Again, the size of the peak is proportional to the
concentration of drug in the sample. HPLC can be used to separate and analyse
complex mixtures.
Applications of HPLC:
·
Preparative
HPLC refers to the process of isolationand purification of
compounds. Important is the degree of solute purity and the throughput, which
is the amount of compound produced per unit time. This differs from analytical HPLC, where the focus is to
obtain informationabout the sample compound. The information that can be
obtained includes identification, quantification, and resolu-tion of a
compound.
·
Chemical
Separations can be accomplished using HPLCby utilising the fact that
certain compounds have different migration rates given a particular column and
mobile phase. Thus, the chromatographer can separate compounds from each other
using HPLC; the extent or degree of separation is mostly determined by the
choice of stationary phase and mobile phase.
· Purification refers to the process of separating or extractingthe target
compound from other (possibly structurally related) compounds or contaminants.
Each compound should have a characteristic peak under certain chromatographic
conditions. Depending on what needs to be separated and how closely related the
samples are, the chromatographer may choose the conditions, such as the proper
mobile phase, to allow adequate separation in order to collect or extract the
desired compound as it elutes from the stationary phase. The migration of the
compounds and contaminants through the column need to differ enough so that the
pure desired compound can be collected or extracted without incurring any other
undesired compound.
· Identification of compounds by HPLC is a crucial partof any HPLC assay. In order to identify any compound by HPLC a detector must first be selected. Once the detector is selected and is set to optimal detection settings, a separa-tion assay must be developed.
The parameters of this assay should be such that a clean peak of the
known sample is observed from the chromatograph. The identifying peak should
have a reasonable retention time and should be well separated from extraneous
peaks at the detection levels which the assay will be performed. To alter the
retention time of a compound, several parameters can be manipulated. The first
is the choice of column, another is the choice of mobile phase, and last is the
choice in flow rate. Identifying a compound by HPLC is accomplished by
researching the literature and by trial and error. A sample of a known compound
must be utilised in order to assure identification of the unknown compound.
Identification of compounds can be assured by combining two or more detection
methods.
· Quantification of compounds by HPLC is the process ofdetermining the
unknown concentration of a compound in a known solution. It involves injecting
a series of known concentrations of the standard compound solution onto the
HPLC for detection. The chromatograph of these known concentrations will give a
series of peaks that correlate to the concentration of the compound injected.
4. Mass Spectrometry (MS)
This is usually combined with gas
chromatography (GC-MS), and is
considered to be the best technique for quantitative analysis of a wide variety
of chemicals, but its expense (capital as well as operational costs) greatly
restricts its use.
In the simplest terms the GC-MS
instrument represents a device that separates chemical mixtures (the GC
component) and a very sensitive detector (the MS component) with a data
collector (the computer component). Once the sample solution is introduced into
the GC inlet it is vapourised immediately because of the high temperature
(250°C) and swept onto the column by the carrier gas (usually helium). The
sample flows through the column experiencing the normal separa-tion processes.
As the various sample components emerge from the column opening, they flow into
the capillary column interface (Fig 36.5).
This device is the connection between the GC column and the MS. Some interfaces
are separators and concentrate the sample via removal of the helium carrier.
The sample then enters the ionisation chamber.
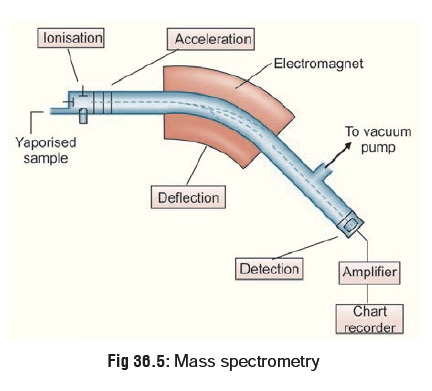
Two potential methods exist for ion production. The most frequently used method for toxicological purposes is electron impact (EI). The occasionally used alternative is chemical ioni-sation (CI). For electron impact ionisation a collimated beam of electrons impact the sample molecules causing the loss of an electron from the molecule. A molecule with one electron missing is represented by M+ and is called the molecular ion (or parent ion). When the resulting peak from this ion is seen in a mass spectrum, it gives the molecular weight of the compound. Chemical ionisation begins with ionisation of methane (or other gas), creating a radical which in turn will impact the sample molecule to produce MH+ molecular ions. Some of the molecular ions fragment into smaller daughter ions and neutral fragments. Both positive and negative ions are formed but only positively charged species will be detected.
Less fragmentation occurs with CI
than with EI, hence CI yields less information about the detailed structure of
a molecule, but does yield the molecular ion; sometimes the molecular ion
cannot be detected by the EI method, hence the two methods complement one
another. Once ionised, a small positive potential is used to repel the positive
ions out of the ionisation chamber.
The next component is a mass
analyser (filter), which sepa-rates the positively charged particles according
to their mass. Several types of separating techniques exist; quadrupole
filters, ion traps, magnetic deflection, time-of-flight, radio frequency,
cyclotron resonance and focusing to name a few. The most common are quadrupoles
and ion traps. After the ions are separated according to their masses, they
enter a detector and then on to an amplifier to boost the signal. The detector
sends information to the computer which acts as a “clearing house”. It records
all the data produced, converts the electrical impulses into visual displays
and hard copy displays. The computer also drives the mass spectrometer.
Identification of a compound based
on it’s mass spectrum relies on the fact that every compound has a unique
frag-mentation pattern. Even isomers can be differentiated by the experienced
operator. Generally, more information is gener-ated than could possibly be
used. A library of known mass spectra which may be several thousand compounds
in size is stored on the computer and may be searched using computer algorithms
to assist the analyst in identifying the unknown. It is important to
incorporate all other available structural informa-tion (chemical, spectral,
sample history) into the interpretation wherever appropriate.
5. Radio Immunoassay (RIA)
It is a slow and expensive method of detecting drugs in the blood, but is highly accurate. It involves mixing known quanti-ties of drug specific antibody with known amount of radioac-tively labelled drug which allows analysis of the precipitate with a gamma counter. The amount of emittance inversely correlates with the presence of assayed drug. This test is excel-lent for detection of drugs in extremely low blood concentrations (cannabis, digoxin, LSD, paraquat, etc.).
Principle:
In
radioimmunoassay (RIA), a fixed concen-tration of labelled tracer antigen is
incubated with a constant amount of antiserum such that the concentration of
antigen binding sites on the antibody is limiting, for example, only 50% of the
total tracer concentration may be bound by antibody. If unlabelled antigen is
added to this system, there is competi-tion between labelled tracer and
unlabelled antigen for the limited and constant number of binding sites on the
antibody, and thus the amount of tracer bound to antibody will decrease as the
concentration of unlabelled antigen increases. This can be measured after
separating antibody-bound from free tracer and counting either the bound
fraction, the free fraction or both. A calibration or standard curve is set up
with increasing amounts of known antigen, and from this curve the amount of
antigen in the unknown samples can be calculated. Thus the four basic
necessities for a radioimmunoassay system are an antiserum to the compound to
be measured, the availability of a radioactively labelled form of the compound,
a method whereby antibody-bound tracer can be separated from unbound tracer,
and a standard unlabelled material.
Radioimmunoassay is widely-used
because of its great sensitivity. Using antibodies of high affinity ( K0
= 108–1011M−1), it is possible to
detect a few picograms (10−12gm) ofantigen in the tube.
6. Enzyme Mediated Immunoassay Technique (EMIT)
This is a fast, expensive method
with good accuracy, which works on the principle that the amount of drug
present is proportional to the inhibition of an enzyme-substrate reaction. A
known quantity of a drug is labelled by chemical attachment to an enzyme. Drug
specific antibodies added to the specimen bind the drug-enzyme complex thereby
reducing enzyme activity. Free drug in the specimen competes with enzyme
labelled drug and limits the antibody-induced enzyme inactivation. Enzyme
activity correlates with drug concentration in the specimen as measured by
absorbance change resulting from the enzyme catalytic action on a substrate.
Enzyme mediated immunoassay technique (EMIT) is preferred over other
radioimmunoassay methods in the emergency situation because of its simplicity
and speed in providing information on toxic drug concentrations (approxi-mately
one sample per minute). It eliminates the complex separation phase necessary in
RIA. There are two types : EMIT-st (single test) which consists of a compact spectro-photometer for
small laboratories, and EMIT-dau(drugs ofabuse) for larger hospitals.
·
The two main disadvantages are:
·
Negative result does not exclude the ingestion of a drug
that may be present in undetectable quantities.
·
Antibody cross-reactions can produce false positive results.
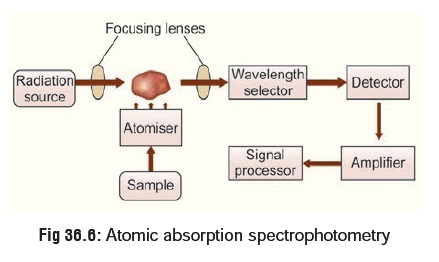
7. Atomic Absorption Spectrophotometry (AAS)
This is the best method for
detecting inorganic elements (arsenic, lead, mercury, thallium, etc.). However
it requires a large sample of blood for accurate analysis. The blood sample is
introduced into a high temperature oxyacetylene flamesituated in the path of a
beam of radiation. The organic matrix is combusted and the metal forms a cloud
of atoms which absorbs a fraction of the radiation in proportion to the
concen-tration of metal in the sample. Light of a suitable wavelength for a
particular element is shone through the flame, and some of this light is
absorbed by the atoms of the sample. The amount of light absorbed is
proportional to the concentration of the element in the solution, and hence in
the original object (Fig36.6).
Measurements are made separately for each element ofinterest in turn to achieve
a complete analysis of an object, and thus the technique is relatively slow to
use. However, it is very sensitive and it can measure trace elements down to
the part per million level, as well as being able to measure elements present
in minor and major amounts.
The equipment is highly complex and
can be operated only by trained personnel. The capital cost is prohibitive.
Flame AAS techniques are the oldest methods, and they can measure
parts-per-million element concentrations with accuracies in the 1–3% range.
Graphite furnace AAS, a more recent develop-ment, uses much smaller samples,
and can make parts-per-billion measurements with about 20% accuracy. The newest
technique, diode laser AAS, can look at several elements in a sample
simultaneously using an array of lasers that emit various wavelengths of light.
A mathematical process is then used to separate the mixture of light
wavelengths that reaches the detector.
Inductively Coupled Plasma Atomic Emission Spectroscopy (ICP-AES) is a new development that allowssimultaneous multi-element
analysis. Seventeen elements can be measured from a single sample : aluminium,
barium, cadmium, chromium, copper, iron, lanthanum, lead, manganese,
molybdenum, nickel, platinum, silver, strontium, tin, titanium, and zinc.
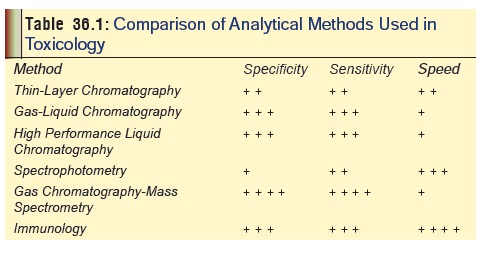
Table 36.1 gives the comparative status of efficiency of thecommonly
used laboratory techniques in toxicology.
8. Neutron Activation Analysis (NAA)
This is a highly sophisticated and expensive method of detec-tion of a variety of inorganic elements at levels well below the limits of conventional analytical techniques. The sensitivity attained is remarkable—sometimes 100 or even 1000 times better than by any other method. It is based on the principle that many substances become radioactive when exposed to bombardment by neutrons. The induced radioactivity is highly specific of the elements contributing to it. Consequently, a study of this radioactivity permits identification and quantitative estimation of many elements.
Radioactivity is a spontaneous disintegration of the atomic nucleus. It occurs
in a number of naturally occurring elements including radium, uranium, and
thorium. Radioactive varieties (isotopes) of all other elements may be made
artificially as explained above, by exposing them to bombardment by neutrons.
However this requires the use of a nuclear reactor, though attack by charged
particles such as protons and deuterons obtained from high energy accelerators
may also be serviceable. Assay of the induced radioactivity can be done by
means of either a Geiger counter or a scintil-lation counter.
Neutron activation analysis is a
sensitive analytical tech-nique useful for performing both qualitative and
quantitative multi-element analysis of major, minor, and trace elements in
samples from almost every conceivable field of scientific or technical
interest. For many elements and applications, NAA offers sensitivities that are
superior to those attainable by other methods, on the order of parts per
billion or better. In addition, because of its accuracy and reliability, NAA is
gener-ally recognised as the “referee method” of choice when new procedures are
being developed or when other methods yield results that do not agree.
Worldwide application of NAA is sowidespread it is estimated that approximately
100,000 samples undergo analysis each year. Neutron activation analysis can be
used for the estimation of any of the 90 naturally occurring elements including
antimony, arsenic, cadmium, copper, iron, lead, mercury, selenium, thallium,
and zinc.
Related Topics