Chapter: Modern Medical Toxicology: Analytical Toxicology: Analytical Instrumentation
Qualitative Tests - Analytical Toxicology Instrumentation
QUALITATIVE TESTS
1. Bedside Tests
a. Colour Tests
–– Trinder’s test: Add 100 ml of Trinder’s
reagent (40 gm mercuric chloride in 850 ml water and 120 ml aqueous
hydrochloric acid mixed with 40 gm hydrated ferric nitrate diluted to 1 litre
with warm water), to 2 ml urine, and mix for 5 seconds.
A
violet or purple colour indicates the presence of salicylates (salicylic acid,
salicylamide, and methyl salicylic acid). If the specimen merely darkens, the
result is considered negative.
If
only stomach contents or scene residues are avail-able, hydrolyse by heating
with 0.5 mol/L hydro-chloric acid in a boiling water bath for 2 minutes, and
neutralise with 0.5 mol/L sodium hydroxide before performing the test.
–– Ferric chloride test: Add 1 ml of 5% ferric chloride solution to
2 ml urine. A persistent purple colour indicates the presence of phenol,
pheno-thiazines, phenylbutazone, oxyphenbutazone, or salicylates.
–– FPN test: Add 1 ml of FPN reagent (a mixture of 5 ml aqueous
ferric chloride, 45 ml aqueous perchloric acid, and 50 ml aqueous nitric acid),
to1 ml of urine or stomach contents and mix for 5 seconds.
Colours ranging from pink, red,
violet, to blue may indicate the presence of phenothiazines. Tricyclics may
give green or blue colour.
–– O-Cresol test: Add 0.5 ml
concentrated hydro-chloric acid to 0.5 ml urine or stomach contents,heat in a
boiling water bath for 10 minutes, and cool. Add 1 ml of aqueous O-cresol
solution (10 gm/L) to 0.2 ml of the hydrolysate, followed by 2 ml ammonium
hydroxide, and mix for 5 seconds.
A blue or blue-black colour
indicates the presence of paracetamol or phenacetin.
Dichromate test: Add equal volumes
of 10% sodium dichromate in 50% sulfuric acid to the urine sample. Development
of green colour indicates the presence of ethanol.
––
Marquis test: Add a mixture of 3 ml concentrated sulfuric acid and 3
drops of formalin to the gastric fluid. A purple colour which gradually turns
blue, indicates the presence of opium or its derivatives.
Lee-Jones test: Add a few crystals
of ferroussulfate and 4 to 5 drops of 2% sodium hydroxide to5 ml gastric fluid.
Boil and cool. Add 8 to 10 drops of 10% hydrochloric acid.
A greenish-blue colour indicates
cyanide, while chloric acid and a small strip of copper. This is purple colour
indicates salicylates in the sample.
––
Reinsch test: 20 ml of stomach contents or urine is placed in a conical
flask along with 10 ml hydro- gently heated for an hour in a boiling water bath
inside a fume cupboard. The copper is then removed and examined. A silvery
deposit indicates mercury or bismuth, and a purplish-black deposit indicates
poisoning, while a black deposit indicates arsenicantimony.
Qualitative desferrioxamine colour test (QDCT): 2 ml of gastric fluid and 2 drops
of 30% hydrogen peroxide are placed in two plastic tubes. 5 ml of
desferrioxamine solution (500 mg in 4 ml of distilled water) is placed in one
tube, and the resulting colour change is compared with the other tube
(control).
If an orange or red colour develops,
it indicates the presence of toxic levels of iron. The test must be done within
2 hours of poisoning or else it is unreliable.
A variation of this test is the Desferrioxaminechallenge test (DCT), in
which 25 to 50 mg/kg ofdesferrioxamine is administered to the patient by
intramuscular injection. Iron poisoning is indicated if the urine which is
voided subsequently is pinkish in colour (vin
rose´urine).
–– Meixner test: This test can be done on either stool or gastric
sample. Dilute the sample with methanol, centrifuge, and filter it. Add a drop
or two to a piece of newspaper. Encircle the spot with a pencil and dry it. Add
a few drops of concentrated hydrochloric acid to the spot.
If a blue colour forms within a few
minutes, it is indicative of the presence of amatoxin (present in most toxic
mushrooms).
–– Forrest test: Add 1 ml Forrest reagent (a mixture of 25 ml
aqueous potassium dichromate, 25 mlaqueous sulphuric acid, 25 ml aqueous
perchloric acid, and 25 ml aqueous nitric acid) to 0.5 ml of the sample, and
mix for 5 seconds. A yellow-green colour deepening to blue indicates the
presence of imipramine or related compounds.![]()
–– Fujiwara test: To three 10 ml tubes, add respec-tively 1 ml
portions of (a) the sample, (b) purified water, and (c) aqueous trichloroacetic
acid (10 mg/L). Add 1 ml sodium hydroxide solution (5 mol/L), and 1 ml pyridine
to each tube, mix care-fully, and heat in a boiling water bath for 2 minutes.
An intense red/purple colour in the top layer of tubeas in tube (c) indicates
the presence of trichloro compounds such as chloral hydrate, chloroform, and
trichloro ethylene. Tube (b) should show no colouration.
b. Other Tests
–– Isonitrile test: Mix a small amount of gastric contents with 10
ml water and add 1 ml purified aniline, followed by 2 ml 20% sodium hydroxide.
Heat gently for a few minutes.
A foul odour (skunk odour) will be perceived if one of the following poisons is
present: carbon tetrachloride, chloral hydrate, chloroform, methyl bromide, or
any other chlorinated hydrocarbon.
–– Tensilon (edrophonium challenge) test: When 10 mg edrophonium is
given intravenously in a case of sudden paralysis, there will be dramatic
recovery if it is due to myasthenia gravis, while a case of poisoning (e.g.
botulism) will not show any improvement.
–– Melzer’s test: This is a test done to confirm whether a given
mushroom is toxic (especially Amanita
phalloides). The spores obtained fromthe mushroom are stained with 1 drop
of Melzer’s reagent (mixture of 20 ml water, 1.5 gm potas-sium iodide, 0.5 gm
iodine, and 20 gm chloral hydrate), and viewed under a microscope. Spores of A. phalloides and a few other deadly
mush-rooms will show a bluish black colour (“amyloid reaction”). However, a
negative reaction does not mean that the mushroom is non-toxic.
2. Thin Layer Chromatography
This is a qualitative technique
which involves the movement by capillary action of a liquid phase (usually an
organic solvent) through a thin, uniform layer of stationary phase (usually
silica gel) held on a rigid support (usually a glass, aluminium, or plastic
sheet). Compounds are separated by partition between the mobile and stationary
phases.
A very thin layer of silica gel or aluminium oxide is applied to a glass plate 20 × 20 × 0.5 cm, and vertical lines are drawn 1.5 cm apart to allow individual runways for each sample. Purified tissue extracts dissolved in 0.5 ml of methanol are serially spotted with a micropipette and dried in a small circle in the lower centre of a runway 1.5 cm from the bottom. Other samples are similarly spotted in other runways. A horizontal line (stop point) is drawn 10 or 15 cm above these startingpoints. A TLC tank is filled with suitable developing solvents to a depth of about 1 cm from the bottom. The plate properly spotted is then dipped into the solvent, the lid is firmly closed and the atmosphere is allowed to saturate with vapour. When the solvent front just touches the 10 cm horizontal mark, quickly remove the plate and examine under UV light (at 254 nm and 366 nm) for characteristic fluorescence or absorbance (Fig 36.1). Calculate the Rf value. This can also be done after spraying the plate with appropriate reagents to bring out char-acteristic colour spots.* This widens the scope of the analysis and increases the confidence of identification.
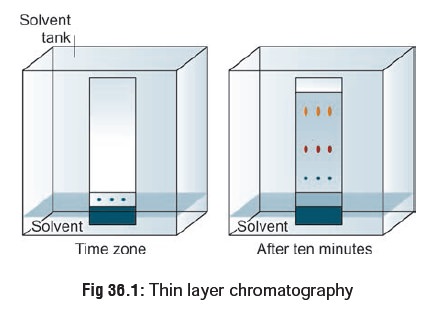
Approximate quantitation can be done
by comparison with standards similarly prepared on the same plate, for
intensity of colour and area size. The Rfs suggested in the literature can be
used as a guide for identifying various poisons, though it is preferable that
each analyst should prepare and establish his own Rf according to his own
conditions and technique.
The
recommended TLC visualisation reagents are as follows:
·
Mercurous nitrate reagent (acidic extract) which gives white
spots with a grey centre on a darker back-ground with barbiturates and related
compounds.
·
Acidified iodoplatinate reagent (basic extract) which gives
mainly purple, blue, or brown spots with a range of basic and neutral drugs and
metabolites.
·
Mandelin’s reagent (basic extract) which gives colours
ranging from blue and green to orange and red with a variety of basic
compounds.
·
Sulfuric acid (500 ml/L)(basic extract) gives red, purple,
or blue spots with many phenothiazines and their metabolites.
It
is to be noted that many additional mobile phase and spray reagent combinations
could be used in place of those suggested here.
Thin
layer chromatography (TLC) is a simple, inexpensive technique which is widely
used. It takes only about 2 hours frombeginning to completion. It is also a
very versatile method since the order of separation of compounds can be altered
simply by changing the nature of the developing agent. However, inter-pretation
of the plates can prove difficult and calls for a trained eye with considerable
experience in recognising colours, spot shapes, and metabolite patterns.
Troubleshooting
·
The compound runs as a streak rather than a spot: The sample
was overloaded. Run the TLC again after diluting the sample. Or, the sample
might just contain many components, creating many spots which run together and
appear as a streak, i.e. the procedure did not go as well as expected. Repeat!
The sample runs as a smear or an upward crescent:
·
Compounds which possess strongly acidic or basic groups
(amines or carboxylic acids) sometimes show up on a TLC plate with this
behaviour. Add a few drops of ammonium hydroxide (amines) or acetic acid
(carboxylic acids) to the eluting solvent to obtain clearer plates.
·
The sample runs as a downward crescent: Likely, the
adsorbent was disturbed during the spotting, causing the crescent shape.
·
The plate solvent front runs crookedly: Either the adsorbent
has flaked off the sides of the plate, or the sides of the plate are touching
the sides of the container (or the paper used to saturate the container) as the
plate develops. Crookedly run plates make it harder to measure Rf values
accurately.
·
No spots are seen on the plate: The operator may not have
spotted enough compound, perhaps because the solution of the compound is too
dilute. This can be resolved by concentrating the solution, or, spotting it
several times in one place, allowing the solvent to dry between applica-tions.
Some compounds do not show up under UV light;try another method of visualising
the plate.
·
If the solvent level in the developing jar is deeper than
the origin (spotting line) of the TLC plate, the solvent will dissolve the
compounds into the solvent reservoir instead of allowing them to move up the
plate by capillary action.
·
In such a case also, spots will not be seen after the plate
is developed.
Related Topics