Chapter: Essential Clinical Immunology: Basic Components of the Immune System
Antibody
ANTIBODY
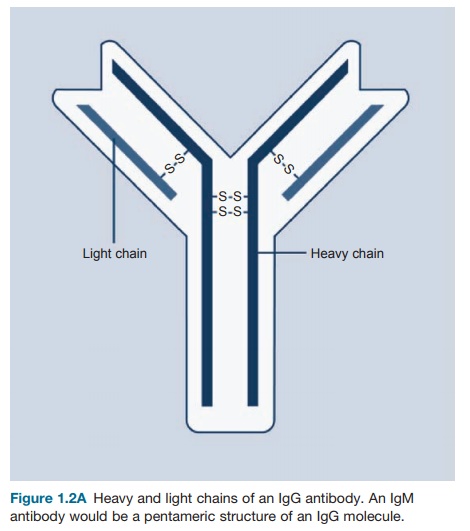
The basic structure of the antibody molecule is
depicted in Figures 1.2A and B. It consists of a four-chain
structure divided into two identical heavy (H) chains with a molecular weight
of 25 kDa. Each chain is composed of domains
of 110 amino acids and is connected in a loop by a disulfide bond between two
cysteine residues in the chain.
The amino
acid N-terminal domains of the heavy and light chains include the
anti-gen-binding site. The amino acids of these variable domains vary between
different antibody molecules and are thus known as the variable (V) regions. Most of these dif-ferences reside in the hypervariable areas of the molecule and
are usually only six to ten amino acid residues in length. When the
hypervariable regions in each chain come together along with the counterparts
on the other pair of H and L chains, they form the antigen-binding site. This
part of the molecule is unique to the molecule and is known as the idiotype determinant. In any individual,
106 to 107 different anti-body molecules can be composed
from 103 different heavy and light chains of the variable regions.
The part of the molecule next to the V region is called the constant(C) region
made up of one domain in the light chain (C1) and three or four in a
heavy chain (CH). A Cl chain may consist of either
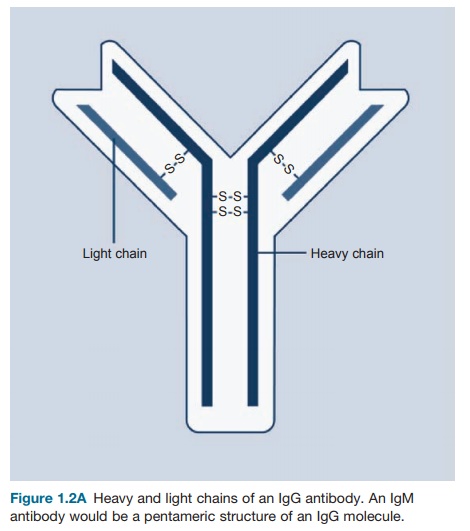
Figure 1.2A Heavy
and light chains of an IgG antibody. An IgM antibody would be a pentameric structure of an IgG molecule.
two kappa (κ) or two lambda (λ) chains but never one of each. Of all the human anti-body molecules, approximately
60%, are κ chains
and 40% contain λ chains.
Although there are no known differences in the func-tional properties of κ and λ chains, there are several
different types of the CH domain. These differences are reflected in
determin-ing the class (isotype) of the antibody and thereby the physiological
function of a par-ticular antibody molecule.
The IgM
molecule is the oldest class of immunoglobulins, and it is a large mol-ecule
consisting of five basic units held together by a J chain. The major role IgM
plays is the intravascular neutralization of organisms, especially viruses. The
reason for this important physiological role is that it contains five
complement-binding sites, resulting in excellent complement activation. This
activation permits the segment removal of antigen–antibody complement complexes
via complement receptors on phagocytic cells or complement-mediated lysis of
the organism. However, in contrast to the IgG molecule, it has relatively low
affinity binding to the antigen in question. Second, because of its size, it
does not usu-ally penetrate into tissues.
In
contrast, IgG is a smaller molecule that penetrates easily into tissues. There
are four major classes of IgG: IgG1 and IgG3 activate
complement efficiently and clear most protein antigens, including the removal
of microorganisms by phago-cytic cells. In contrast, IgG2 and IgG4
react mostly with carbohydrate antigens and are relatively poor opsonins. This
is the only molecule that crosses the placenta to pro-vide immune protection to
the neonate.
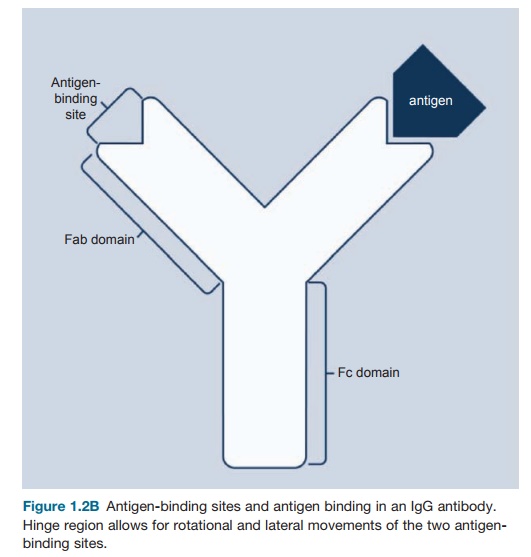
Figure 1.2B Antigen-binding
sites and antigen binding in an IgG antibody. Hinge region allows for rotational and lateral movements of
the two antigen-binding sites.
The major mucosal immunoglobulin,
IgA, consists of two basic units joined by a J chain. The addition of a
secretion molecule prevents its digestion by enzymes present in mucosal and
intestinal secretions. Thus, IgA2 is the major IgA molecule in
secretions and is quite effective in neutralizing anti-gens that enter via
these mucosal routes. IgA1, the main IgA molecule in serum, is,
however, susceptible to inactivation by serum proteases and is thus less active
for defense. Its function is unclear at present.
Two other
classes are worthy of note. IgD is synthesized by antigen-sensitive B cells and
is involved in the activation of these cells by antigen. IgE is produced by
plasma cells and binds to specific IgE recep-tors on most cells and basophiles.
This molecule plays an extremely
important
role in allergic reactions and expelling intestinal parasites, which is
accomplished by increasing vascular per-meability and inducing chemotactive
fac-tors following mast cell degranulation
Given
this extraordinary ability to gen-erate large numbers of antibody molecules,
how does the immune system recognize all pathogens, including past, present,
and future? This diversity is achieved by the way in which the genetics of
antibody production is arranged (see Figure 1.3). The light and heavy chains
are carried on different chromosomes. The heavy chain genes are carried on
chromosome 14. These genes are broken up into
coding systems called exons with
intervening segments of silent segments called entrons. The exons represent the central region of the heavy
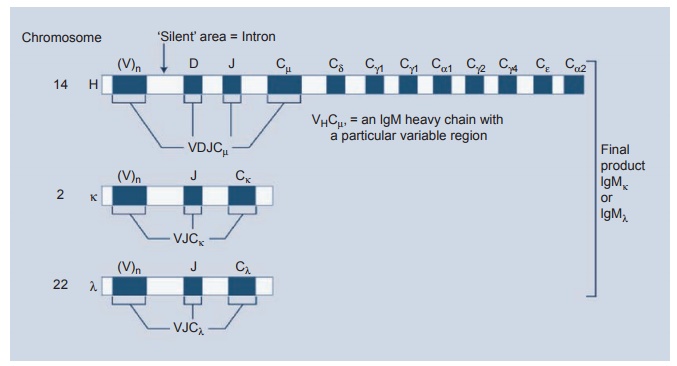
Figure 1.3 The
genetics of antibody production
chain and
a large number of V regions. Between the V and D genes are two small sets of
exons called the D and J. With each single B cell, one V gene is joined to one
D and J in the chromosome. The product, the VH domain, is then
joined at the level of RNA processing to Cu and the B cell makes an
IgM molecule. By omitting the Cu gene and joining VHDJ
to a Cλ an IgG
molecule is produced. This enormous versatility allows the cell to make IgM,
IgD, IgG, IgA, or Ig in sequence while using the same variable regions (see
Figure 1.4). The heavy chain gene recombinations are controlled by two
recombination activity genes called RAG1 and RAG2. If
these genes are eliminated by “knock-out” techniques in mice, profound
immunodeficiency status occurs in these animals, characterized by absent mature
B and T cells.
Thus, the
diversity of antigen bind-ing is achieved by the large number of V
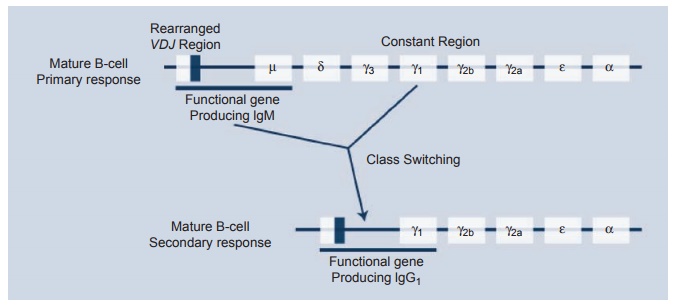
Figure 1.4 Recombination
events necessary for generation of class and subclass switching.
genes available and their combination
with different D and L genes to provide differ-ent antibodies. Furthermore, the
inherited set of genes may be increased by somatic mutation during multiple
divisions of lym-phoid cells, thereby increasing the number of antibody
specificities to 1014, which far exceeds the number of B cells (1010)
in the body.
Once a
given B cell is preselected to pro-duce a particular VH and VL
domain, all the ensuing progeny of that B cell will produce the same VH
or VL domain. The sequence of events is as follows: initially, the B
cell produces intracellular antigen-specific IgM, which becomes bound to the
cell sur-face. The B cell is now antigen responsive with exposure to a given
antigen. The com-mitted B cell begins producing a certain isotype or class of
immunoglobulins and begins dividing, and all the progeny will produce the
identical immunoglobulin mol-ecules. These B cells will later mature into
either plasma cells or long-term memory B cells.
Related Topics