Chapter: Clinical Anesthesiology: Anesthetic Management: Anesthesia for Neurosurgery
Anesthesia for Surgery in the Posterior Fossa
Anesthesia for Surgery in the Posterior Fossa
Craniotomy for a mass in the posterior fossa pres-ents a unique
set of potential problems: obstructive hydrocephalus, possible injury to vital
brainstem centers, pneumocephalus, and, with unusual posi-tioning, postural
hypotension and venous air
embolism.
Obstructive Hydrocephalus
Infratentorial masses can obstruct CSF
flow through the fourth ventricle or the cerebral aqueduct of Sylvius. Small
but critically located lesions can mark-edly increase ICP. In such cases, a
ventriculostomy is often performed under local anesthesia to decrease ICP prior
to induction of general anesthesia.
Brain Stem Injury
Operations in the posterior fossa can
injure vital circulatory and respiratory brainstemcenters and cranial nerves or
their nuclei. Such inju-ries may occur as a result of direct surgical trauma or
ischemia from retraction or other interruptions of the blood supply. Damage to
respiratory centers is said to nearly always produce circulatory changes;
therefore, abrupt changes in blood pressure, heart rate, or cardiac rhythm
should alert the anesthesiolo-gist to the possibility of such an injury. Such
changes should be communicated to the surgeon. Isolated damage to respiratory
centers may rarely occur with-out premonitory circulatory signs during
operations in the floor of the fourth ventricle. Historically, some clinicians
have employed spontaneous ventilation during these procedures as an additional
monitor of brain function. At completion of the surgery, brain-stem injuries
may present as an abnormal respiratory pattern or an inability to maintain a
patent airway following extubation. Monitoring brainstem audi-tory evoked
potentials may be useful in preventing eighth nerve damage during resections of
acoustic neuromas. Electromyography is also used to avoid injury to the facial
nerve, but requires incomplete neuromuscular blockade intraoperatively.
Positioning
Although most explorations of the posterior fossa can be
performed with the patient in either a modi-fied lateral or prone position, the
sitting position may be preferred by some surgeons.
The patient is actually semirecumbent in the standard sitting
position ( Figure27–1);
the back is elevated to 60°, and the legs are elevated with the knees flexed.
The head is fixed in a three-point holder with the neck flexed; the arms remain
at the sides with the hands resting on the lap.Careful positioning and padding
helps avoid injuries. Pressure points, such as the elbows, ischial spines,
heels, and forehead, must be pro-tected. Excessive neck flexion has been
associated with swelling of the upper airway (due to venous
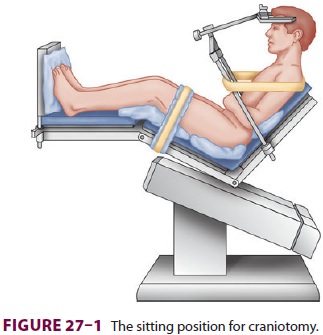
obstruction), and, rarely, quadriplegia (due to com-pression of
the cervical spinal cord). Preexisting cer-vical spinal stenosis probably
predisposes patients to the latter injury.
Pneumocephalus
The sitting position increases the likelihood of
pneu-mocephalus. In this position, air readily enters the subarachnoid space,
as CSF is lost during surgery. In patients with cerebral atrophy, drainage of
CSF is marked; air can replace CSF on the surface of the brain and in the
lateral ventricles. Expansion of a pneumocephalus following dural closure can
com-press the brain. Postoperative pneumocephalus can cause delayed awakening
and continued impairment of neurological function. Because of these concerns,
nitrous oxide is rarely used for sitting craniotomies. (see also below).
Venous Air Embolism
Venous air embolism can occur when the
pressure within an open vein is subatmospheric. These conditions may exist in
any position (and during any procedure) whenever the wound is above the level
of the heart. The incidence of venous air embolism is greater during sitting
craniotomies (20% to 40%) than in craniotomies in any otherposition. Entry into
large cerebral venous sinuses increases the risk.
The physiological consequences of venous air embolism depend on
the volume and the rate of air entry and whether the patient has a
right-to-left intra-cardiac shunt (eg, patent foramen ovale [10% to 25%
incidence]). The latter are important because they can facilitate passage of
air into the arterial circulation (paradoxical air embolism). Modest quantities of air bubbles entering the venous system
ordinarily lodge in the pulmonary circulation, where they are eventu-ally
absorbed. Small quantities of embolized air are well tolerated by most
patients. When the amount entrained exceeds the rate of pulmonary clear-ance,
pulmonary artery pressure rises progressively. Eventually, cardiac output
decreases in response to increases in right ventricular afterload. Preexisting
cardiac or pulmonary disease enhances the effects of venous air embolism;
relatively small amounts of air may produce marked hemodynamic changes. Nitrous
oxide, by diffusing into the bubbles and increasing their volume, can markedly
accentuate the effects of even small amounts of entrained air. The dose for
lethal venous air embolism in animals receiving nitrous oxide anesthesia is
one-third to one-half that of control animals not receiving nitrous oxide.
Definitive signs of venous air embolism are often not apparent
until large volumes of air have been entrained. A decrease in end-tidal CO 2 or arterial oxygen
saturation might be noticed prior to hemodynamic changes. Arterial blood gas
val-ues may show only slight increases in Paco2 as a result of increased pulmonary dead space (areas with
normal ventilation but decreased perfusion). Conversely, major hemodynamic
manifestations, such as sudden hypotension, can occur well before hypoxemia is
noted. Moreover, large amounts of intracardiac air impair tricuspid and
pulmonic valve function and can produce sudden circulatory arrest by
obstructing right ventricular outflow.
Paradoxic air embolism can result in a stroke or coronary
occlusion, which may be apparent only postoperatively. Paradoxic air emboli are
more likely to occur in patients with right-to-left intracardiac shunts,
particularly when the nor-mal transatrial (left > right) pressure gradient is reversed. Some studies suggest that
a right > left pressure gradient can develop at some
time dur-ing the cardiac cycle, even when the overall mean gradient remains
left > right.
A. Central Venous Catheterization
A properly positioned central venous catheter can be used to
aspirate entrained air, but there is only limited evidence that this influences
outcomes after venous air embolism. Some clinicians have consid-ered right
atrial catheterization mandatory for sit-ting craniotomies, but this is a
minority viewpoint.
Optimal recovery of air following venous
air embolism is provided by a multiorificed catheter positioned at the junction between the right
atrium and the superior vena cava. Confirmation of correct catheter positioning
can be accomplished by intravascular electrocardiography, radiography, or
transesophageal echocardiography (TEE). Intra-vascular electrocardiography is
accomplished by using the saline-filled catheter as a “V” lead. Correct high
atrial position is indicated by the appearance of a biphasic P wave. If the
catheter is advanced farther into the heart, the P wave changes from a biphasic
to a undirectional deflection. A right ventricular or pulmonary artery waveform
may also be observed when the catheter is connected to a pressure transducer
and advanced too far.
B. Monitoring for Venous Air Embolism
The most sensitive monitors available should be used. Detecting
even small amounts of venous air embo-lism is important because it allows
surgical control of the entry site before additional air is entrained.
Currently, the most sensitive intraoperative moni-tors are TEE and precordial
Doppler sonography. These monitors can detect air bubbles as small as 0.25 mL.
TEE has the added benefit of detecting the volume of the bubbles and any
transatrial passage through a patent foramen ovale, as well as evaluating any
effect venous air embolism may have on cardiac function. Doppler methods employ
a probe over the right atrium (usually to the right of the sternum and between
the third and sixth ribs). Interruption of the regular swishing of the Doppler
signal by spo-radic roaring sounds indicates venous air embolism. Changes in
end-tidal respiratory gas
concentrations are less sensitive but are important monitors that can also
detect venous air embolism before overt clini-cal signs are present. Venous air
embolism causes a sudden decrease in end-tidal CO2 tension in pro-portion to
the increase in pulmonary dead space; however, decreases can also be seen with
hemody-namic changes unrelated to venous air embolism, such as decreased
cardiac output. A reappearance (or increase) of nitrogen in expired gases may
also be seen with venous air embolism. Changes in blood pressure and heart
sounds (“mill wheel” murmur) are late manifestations of venous air embolism.
C. Treatment of Venous Air Embolism
·
The surgeon should be
notified so that he or she can flood the surgical field with saline or pack it
with wet gauzes and apply bone wax to the skull edges until the entry site is
identified and occluded.
·
Nitrous oxide (if
used) should be discontinued, and the inhalation anesthetic should be delivered
in 100% oxygen.
·
If a central venous
catheter is present, it should be aspirated in an attempt to retrieve the
entrained air.Intravascular volume infusion should be given to increase central
venous pressure.Vasopressors should be given to treat hypotension.
·
Bilateral jugular vein
compression, by increasing cranial venous pressure, may slow air entrainment
and cause back bleeding, which might help the surgeon identify the entry point
of the embolus.
·
Some clinicians
advocate PEEP to increase cerebral venous pressure; however, reversal of the
normal transatrial pressure gradient may promote paradoxic embolism in a
patient with incomplete closure of the foramen ovale.
·
If the above measures
fail, the patient should be placed in a head-down position, and the wound
should be closed quickly.
·
Persistent circulatory
arrest necessitates the supine position and institution of resuscitation efforts
using advanced cardiac life support algorithms.
Related Topics