Chapter: Clinical Anesthesiology: Anesthetic Management: Anesthesia for Neurosurgery
Anesthesia & Craniotomy for Intracranial Aneurysms & Arteriovenous Malformations
Anesthesia & Craniotomy for Intracranial Aneurysms &
Arteriovenous Malformations
Saccular aneurysms and AVMs are common causes of nontraumatic
intracranial hemorrhages. Surgical or interventional neuroradiologic treat-ment
may be undertaken either electively to pre-vent hemorrhage or emergently to
prevent further complications once hemorrhage has taken place. Other
nontraumatic hemorrhages (eg, from hyper-tension, sickle cell disease, or
vasculitis) are usually treated medically.
CEREBRAL ANEURYSMS
Preoperative Considerations
Cerebral aneurysms typically occur at the bifurca-tion of the
large arteries at the base of the brain; most are located in the anterior
circle of Willis. Approximately 10% to 30% of patients have more than one
aneurysm. The general incidence of sac-cular aneurysms in some estimates is
reported to be 5%, but only a minority of those with aneurysms will have
complications. Rupture of a saccular aneurysm is the most common cause of
subarach-noid hemorrhage. The acute mortality following rupture is
approximately 10%. Of those that sur-vive the initial hemorrhage, about 25% die
within 3 months from delayed complications. Moreover, up to 50% of survivors
are left with neurological deficits. As a result, the emphasis in management is
on prevention of rupture. Unfortunately, most patients present only after
rupture has already occurred.
Unruptured Aneurysms
Patients may present with prodromal symptoms and signs
suggesting progressive enlargement. The most common symptom is headache, and
the most common physical sign is a third-nerve palsy. Other manifestations
could include brainstem dysfunction, visual field defects, trigeminal nerve
dysfunction, cavernous sinus syndrome, seizures, and hypothalamic–pituitary
dysfunction. The most commonly used techniques to diagnose an aneu-rysm are MRI
angiography, angiography, and heli-cal CT angiography. Following diagnosis,
patients are brought to the operating room, or more likely the radiology suite,
for elective clipping or oblit-eration of the aneurysm. Most patients are in
the 40- to 60-year-old age group and in otherwise good health.
Ruptured Aneurysms
Ruptured aneurysms usually present
acutely as sub-arachnoid hemorrhage. Patients typically complain of a sudden
severe headache without focal neuro-logical deficits, but often associated with
nausea and vomiting. Transient loss of consciousness may
occur and may result from a sudden rise in ICP and precipitous
drop in CPP. If ICP does not decrease rapidly after the initial sudden
increase, death usu-ally follows. Large blood clots can cause focal
neu-rological signs in some patients. Minor bleeding may cause only a mild
headache, vomiting, and nuchal rigidity. Unfortunately, even minor bleed-ing in
the subarachnoid space seems to predispose to delayed complications. The
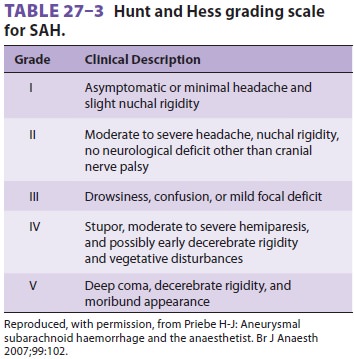
severity of subarach-noid hemorrhage (SAH) is graded according to the Hunt and
Hess scale (Table27–3),
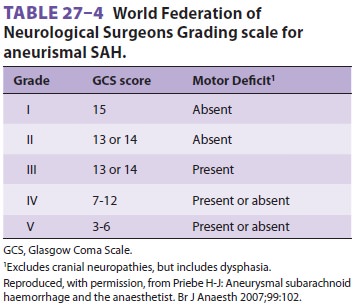
as well as the World Federation of Neurological Surgeons Grading Scale of SAH (
Table27–4).
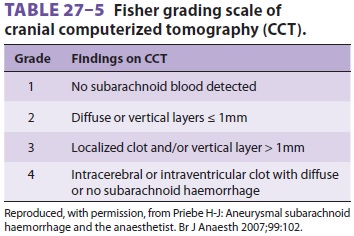
The Fisher grading scale, which uses CT to assess the amount of blood detected,
gives the best indication of the likeli-hood of the development of cerebral
vasospasm and patient outcome ( Table27–5).
Delayed complications include cerebral vaso-spasm, rerupture,
and hydrocephalus. Cerebral vasospasm occurs in 30% of patients (usually after
4–14 days) and is a major cause of morbidity and mortality. Manifestations of
vasospasm are due to cerebral ischemia and infarction and depend on the
severity and distribution of the involved ves-sels. The Ca2+ channel antagonist
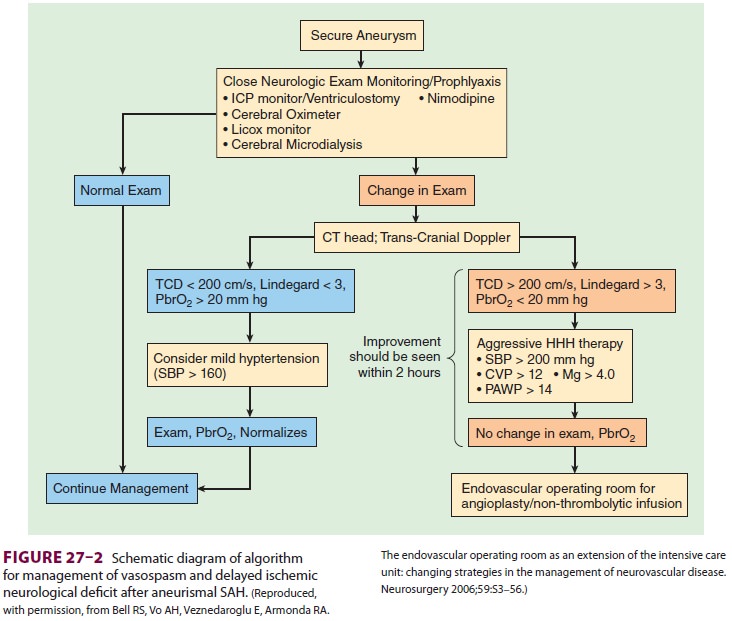
nimodipine may antagonize vasospasm. Both transcranial Doppler and brain tissue
oxygen monitoring can be used to
guide vasospasm therapy (Figure27–2). Increased velocity of flow >200cm/sec is indicative of severe spasm. The Lindegaard ratio
compares the blood velocity of the cervical carotid artery with that of the
middle cerebral artery. A ratio >3 is likewise indica-tive of severe spasm.
Brain tissue oxygen tension less than 20 mm Hg is also worrisome. In patients
with symptomatic vasospasm with an inadequate response to nimodipine,
intravascular volume expansion and induced hypertension (“triple H” therapy:
hypervolemia, hemodilution, and hyper-tension) are added as part of the
therapeutic regi-men. Refractory vasospasm may be treated with infusion of
papaverine, infusion of nicardipine, or angioplasty. However, radiologic improvement in the vessel diameter does not
necessarily correlate with an improvement in clinical status.
PREOPERATIVE MANAGEMENT
In addition to assessing neurological
findings, the preoperative evaluation should include a search for coexisting
diseases, such as hypertension and renal, cardiac, or ischemic cerebrovascular
disease. Electrocardiographic abnormalities are commonly seen in patients with
subarachnoid hemorrhage, but do not necessarily reflect underlying heart
dis-ease. However, increases of cardiac troponin during SAH are associated with
myocardial injury and may herald a poor outcome. Most conscious patients with
normal ICP are sedated following rupture to prevent rebleeding; such sedation
should be con-tinued until induction of anesthesia. Patients with persistent
elevation in ICP should receive little or no premedication to avoid
hypercapnia.
INTRAOPERATIVE MANAGEMENT
Aneurysm surgery can result in exsanguinating hemorrhage as a
consequence of rupture or rebleed-ing. Blood should be available prior to the
start of these operations.
Regardless of the anesthetic technique
emplo-yed, anesthetic management should focus on pre-venting rupture (or
rebleeding) and avoiding factors that promote cerebral ischemia or vasospasm.
Intraarterial and central venous
pressure monitoring are useful. Sudden increases in blood pressure with
tracheal intubation or surgical stimulation should be avoided. Judicious
intravascular volume loading permits surgical levels of anesthesia without
exces-sive decreases in blood pressure. Because calcium channel blockers, angiotensin
receptor blockers, and ACE inhibitors cause systemic vasodilation and reduce
systemic vascular resistance, patients receiv-ing these agents preoperatively
may be particularly prone to hypotension. Hyperventilation is unlikely to
overcome ischemia-induced vasodilation. Once the dura is opened, mannitol is
often given to facili-tate surgical exposure and reduce the need for surgi-cal
retraction. Rapid decreases in ICP prior to dural opening may promote
rebleeding by removing a tamponading effect on the aneurysm.
Elective (controlled) hypotension has been used in aneurysm
surgery. Decreasing mean arte-rial blood pressure reduces the transmural
tension across the aneurysm, making rupture (or rebleed-ing) less likely and
facilitating surgical clipping. Controlled hypotension can also decrease blood
loss and improve surgical visualization in the event of bleeding. The
combination of a slightly head-up position with a volatile anesthetic enhances
the effects of any of the commonly used hypotensive agents. Should accidental
rupture of the aneurysm occur, the surgeon may request transient hypoten-sion
to facilitate control of the bleeding aneurysm.
Technical improvements in temporary vascular clips have enabled
surgeons to use them more often to interrupt blood flow during aneurysm
surgery; induced hypertension is often requested when tem-porary clips are
applied. Neurophysiologic monitor-ing may be employed during aneurysm surgery
to identify potential ischemia during temporary clip application.
Mild hypothermia has been used to
protect the brain during periods of prolonged or excessive hypo-tension or
vascular occlusion; however, its efficacy has been questioned. Rarely,
hypothermic circula-tory arrest is used for large basilar artery aneurysms.
Depending on neurological condition,
most patients should be extubated at the end of surgery. Extubation should be
handled similarly to other craniotomies (see above). A rapid awakening allows
neurological evaluation in the operating room, prior to transfer to the intensive
care unit.
The anesthetic concerns of patients taken for aneursymal coiling
in the neurointerventional suite are similar to those of surgical
interventions. General anesthesia is employed. Patients require heparin
anticoagulation and radiologic contrast. Communication with the surgeon or
neuroradi-ologist as to the desired activated clotting time and need for
protamine reversal is essential. Moreover, anesthesia staff in the
neuroradiology suite must be prepared to manipulate and monitor the blood pres-sure,
as with an open surgical procedure.
ARTERIOVENOUS MALFORMATIONS
AVMs cause intracerebral hemorrhage more often than subarachnoid
hemorrhage. These lesions are developmental abnormalities that result in
arte-riovenous fistulas; they typically grow in size with time. AVMs may
present at any age, but bleed-ing is most common between 10 and 30 years of
age. Other common presentations include head-ache and seizures. The combination
of high blood flow with low vascular resistance can rarely result in high-output
cardiac failure. Acutely, neurora-diologists try to embolize AVMs. When
neuro-radiological interventions are not successful or available, surgical
excision may be undertaken. Neuroradiological embolization employs various
coils, glues, and balloons to obliterate the AVM. Risks include embolization
into cerebral arteries feeding the normal brain, as well as systemic or
pulmonary embolism.
Anesthetic management of patients
undergoing surgical treatment of AVMs may be complicated by extensive blood loss.
Venous access with multiple large-bore cannulas is necessary. Embolization may
be carried out prior to surgery to reduce operative blood loss.
Hyperventilation and mannitol may be used to facilitate surgical access.
Hyperemia and swelling can develop following resection, possibly because of
altered autoregulation in the remaining normal brain. Emergence hypertension is
typically controlled using β1-blockers to avoid any vasodilator induced increase
in CBF.
Related Topics