Chapter: Basic & Clinical Pharmacology : Drugs of Abuse
Addiction: A Disease of Maladaptive Learning
ADDICTION: A DISEASE OF MALADAPTIVE
LEARNING
Addiction is characterized by a high motivation to obtain and use a drug despite negative consequences. With time, drug use becomes compulsive (“wanting without liking”). Addiction is a recalcitrant, chronic, and stubbornly relapsing disease that is very difficult to treat.
The central problem is that even after successful withdrawal and prolonged drug-free periods, addicted individuals have a high risk of relapsing. Relapse is typically triggered by one of the following three conditions: reexposure to the addictive drug, stress, or a con-text that recalls prior drug use. It appears that when paired with drug use, a neutral stimulus may undergo a switch and motivate (“trigger”) addiction-related behavior. This phenomenon may involve synaptic plasticity in the target nuclei of the mesolimbic projection (eg, nucleus accumbens). Several recent studies suggest that the recruitment of the dorsal striatum is responsible for the compulsion.
This switch may depend
on synaptic plasticity in the nucleus accumbens of the ventral striatum, where
mesolimbic dop-amine afferents and cortical glutamatergic afferents converge.
If dopamine release codes for the prediction error of reward (see Box: The
Dopamine Hypothesis of Addiction), pharmacologic stimula-tion of the mesolimbic
dopamine systems will generate an unusu-ally strong learning signal. Unlike
natural rewards, addictive drugs continue to increase dopamine even when reward
is expected. Such overriding of the prediction error signal may eventually be
respon-sible for the usurping of memory processes by addictive drugs.
The
involvement of learning and memory systems in addiction is also suggested by
clinical studies. For example, the role of con-text in relapse is supported by
the report that soldiers who became addicted to heroin during the Vietnam War
had significantly bet-ter outcomes when treated after their return home,
compared with addicts who remained in the environment where they had taken the
drug. In other words, cravings may recur at the presentation of contextual cues
(eg, people, places, or drug paraphernalia). Current research therefore focuses
on the effects of drugs on asso-ciative forms of synaptic plasticity, such as
long-term potentiation (LTP), which underlie learning and memory (see Box:
Synaptic Plasticity & Addiction).
Non-substance-dependent
disorders, such as pathologic gam-bling and compulsive shopping, share many
clinical features of addiction. Several lines of arguments suggest that they
also share the underlying neurobiologic mechanisms. This conclusion is
supported by the clinical observation that, as an adverse effect of dopamine
agonist medication, patients with Parkinson’s disease may become pathologic
gamblers (see Case Study). Other patients may develop a habit for recreational
activities, such as shopping, eating compulsively, or becoming excessively
involved in sexual activity (hypersexuality). Although large-scale studies are
not yet available, an estimated 1 of 7 parkinsonian patients develops an
addiction-like behavior when receiving dopamine agonists.
Large
individual differences exist also in vulnerability to substance-related
addiction. Whereas one person may become “hooked” after a few doses, others may
be able to use a drug occasionally during their entire lives without ever
having difficulty in stop-ping. Even when dependence is induced with chronic
expo-sure, only a small percentage of dependent users progress to addiction.
Recent studies in rats suggest that impulsivity or exces-sive anxiety may be
crucial traits that represent a risk for addic-tion. The transition to
addiction is determined by a combination of environmental and genetic factors.
Heritability of
addiction, as determined by comparing monozygotic with dizygotic twins, is
relatively modest for cannabinoids but very high for cocaine. It is of interest
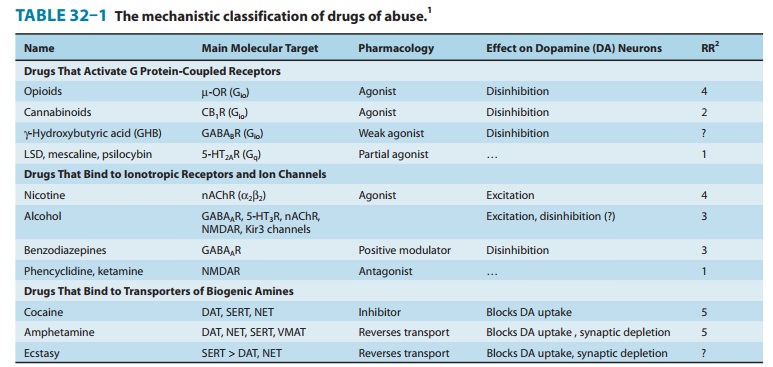
that the relative risk for addiction (addiction liability) of a drug (Table
32–1) correlates with its heritability, suggesting that the neurobiologic basis
of addiction common to all drugs is what is being inherited. Further genomic
analysis indicates that only a few alleles (or perhaps even a single recessive
allele) need to function in combination to produce the phenotype. However,
identification of the genes involved remains elusive. Although some
substance-specific candidate genes have been identified (eg, alcohol
dehydrogenase), future research will also focus on genes implicated in the
neurobiologic mechanisms common to all addictive drugs.
Synaptic Plasticity & Addiction
Long-term potentiation (LTP) is a form of experience-dependent synaptic plasticity that is induced by activating glutamate recep-tors of the N-methyl-D-aspartate (NMDA) type. Since NMDA receptors are blocked by magnesium at negative potentials, their activation requires the concomitant release of glutamate (presyn-aptic activity) onto a receiving neuron that is depolarized (post-synaptic activity). Correlated pre- and postsynaptic activity durably enhances synaptic efficacy and triggers the formation of new connections. Because associativity is a critical component, LTP has become a leading candidate mechanism underlying learning and memory. LTP can be elicited at glutamatergic syn-apses of the mesolimbic reward system and is modulated by dopamine. Drugs of abuse could therefore interfere with LTP atsites of convergence of dopamine and glutamate projections (eg, ventral tegmental area [VTA], nucleus accumbens, or prefrontal cortex). Interestingly, exposure to an addictive drug triggers a specific form of synaptic plasticity at excitatory afferents (drug-evoked synaptic plasticity) and reduces GABAA receptor-mediated inhibition of the VTA. As a consequence, the excitability of dop-amine neurons is increased, the synaptic calcium sources altered, and the rules for subsequent LTP inverted. Genetic manipulations in mice that prevent drug-evoked plasticity at this synapse also have effects on persistent changes of drug-associated behavioral paradigms such as reinstatement of conditioned place prefer-ence, further supporting the idea that synaptic plasticity is involved in context-dependent components of relapse.
Related Topics