Chapter: Essentials of Anatomy and Physiology: Lymphatic System and Immunity
Adaptive Immunity
ADAPTIVE IMMUNITY
Adaptive immunity exhibits specificity and memory. As explained earlier, specificity is the ability to recognize a particular substance, and memory is the ability to respond with increasing effectiveness to successive exposures to the antigen. Antigens (an′ ti-jenz; anti (body) + -gen, producing) are substances that stimulate adaptive immune responses. Antigens can be divided into two groups: foreign antigens and self-antigens.
Foreign antigens are introduced from outside the body.Microorganisms, such as bacteria and viruses, and chemicals released by microorganisms are examples of foreign antigens. Pollen, animal hairs, foods, and drugs can cause an allergic reaction because they are foreign antigens that produce an overreactionof the immune system (see the Diseases and Disorders table). Transplanted tissues and organs contain foreign antigens, and the response to these antigens can cause rejection of the transplant.
Self-antigens are molecules the body produces to stimulatean immune system response. The response to self-antigens can be beneficial. For example, the recognition of tumor antigens can result in destruction of the tumor. But the response to self-antigens can also be harmful. Autoimmune disease results when self-antigens stimulate unwanted destruction of normal tissue. An example is rheumatoid arthritis, which destroys the tissue within joints.
Adaptive immunity can be divided into antibody-mediated immunity and cell-mediated immunity. Antibody-mediatedimmunity involves a group of lymphocytes called B cells andproteins called antibodies(an′ tĕ-bod-ēz), which are found in the plasma. Antibodies are produced by plasma cells, which are derived from the B cells. Cell-mediated immunity involves the actions of a second type of lymphocyte, called T cells. Several subpopulations of T cells exist. For example, cytotoxic (sı̄-tō-tok′ sik; destructive to cells) T cells produce the effects of cell-mediated immunity, and helper T cells can promote or inhibit the activities of both antibody-mediated immunity and cell-mediated immunity.
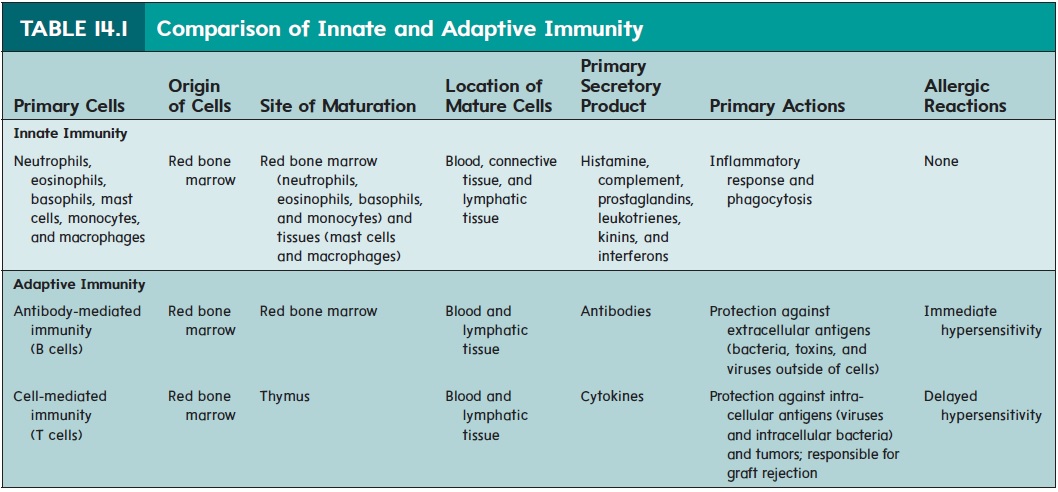
Table 14.1 summarizes and contrasts the main features of innate immunity and the two categories of adaptive immunity (i.e., antibody-mediated immunity and cell-mediated immunity).
Origin and Development of Lymphocytes
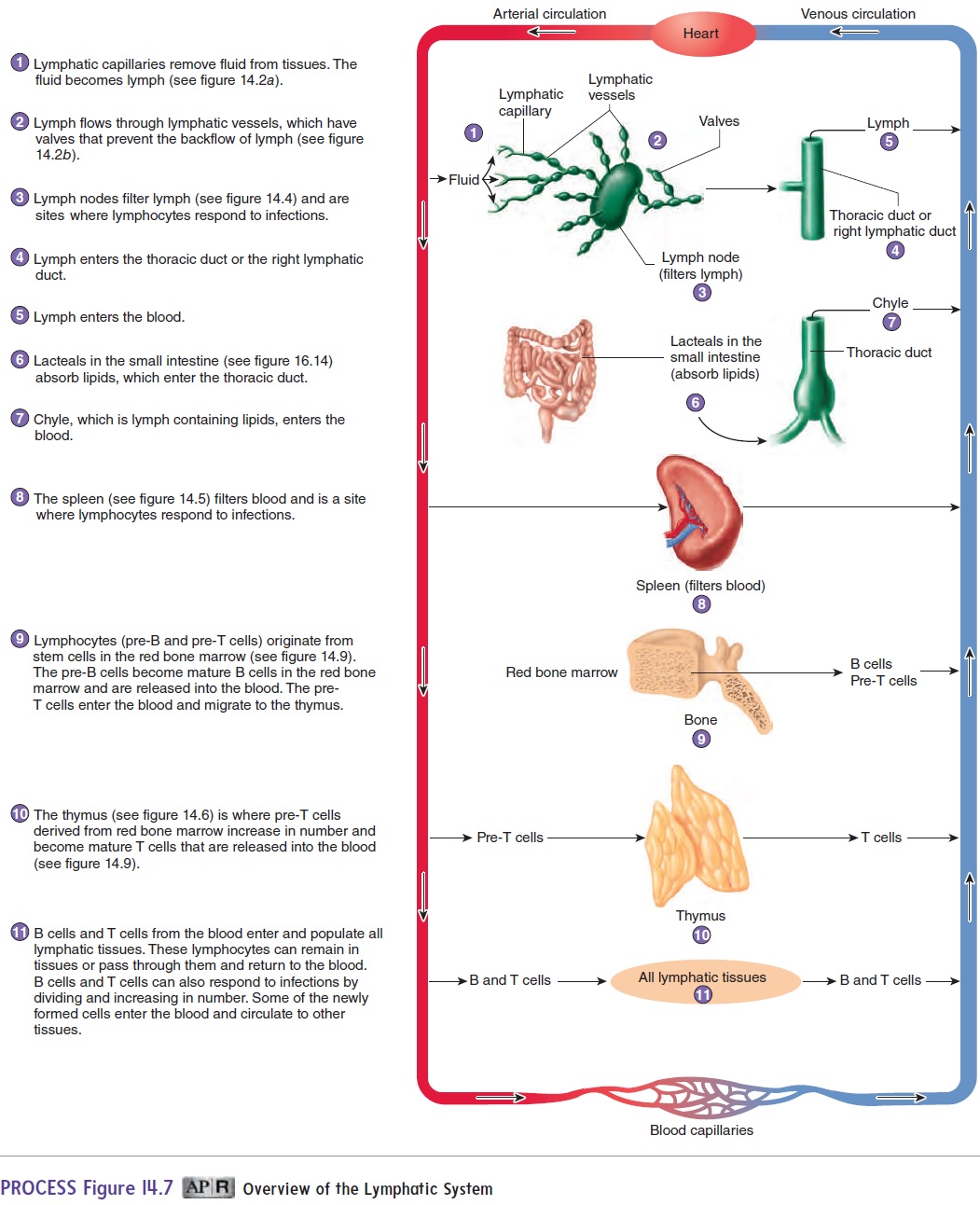
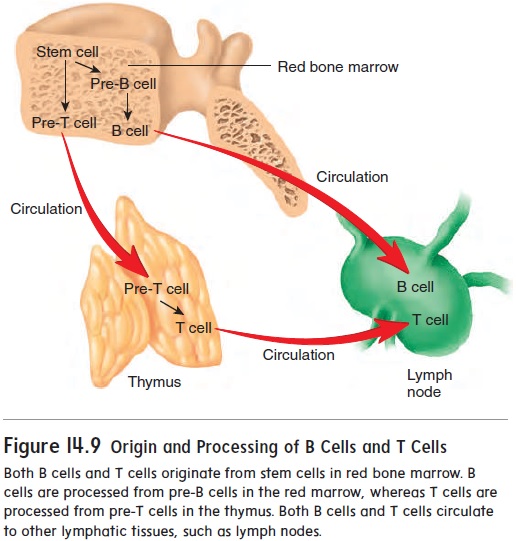
To understand how lymphocytes are responsible for antibody-mediated and cell-mediated immunity, it is important to know how lymphocytes originate and become specialized immune cells. Stem cells in red bone marrow are capable of giving rise to all theblood cells (see figure 11.2). Some stem cells give rise to pre-T cells, which migrate through the blood to the thymus, where they divide and are processed into T cells (figure 14.9). Other stem cells produce pre-B cells, which are processed in the red bone marrow into B cells.
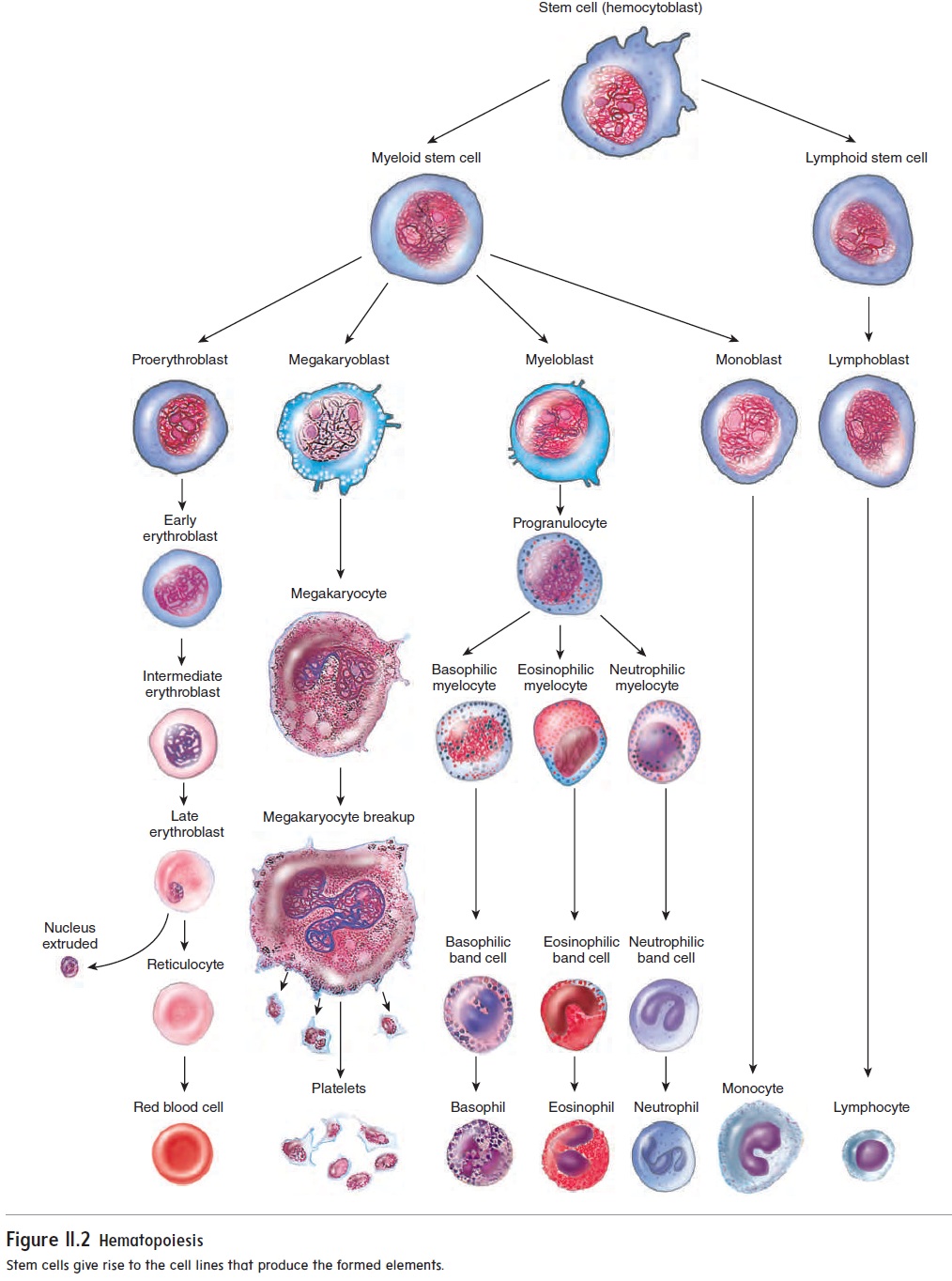
B cells are released from red bone marrow, and T cells are released from the thymus. Both types of cells move through the blood to lymphatic tissues (see figure 14.7). These lymphocytes live for a few months to many years and continually circulate between the blood and the lymphatic tissues. Normally, there are about five T cells for every B cell in the blood. When stimulated by an antigen, B cells and T cells divide, producing cells that are responsible for the destruction of antigens.
Small groups of identical B cells or T cells, called clones, form during embryonic development. Each clone is derived from a single, unique B cell or T cell. Each clone can respond only to a particular antigen. However, there is such a large variety of clones that the immune system can react to most antigens. Among the antigens to which the clones can respond are self-antigens. Because this response could destroy the body’s own cells, clones acting against self-antigens are normally eliminated or suppressed. Most of this process occurs during prenatal development, but it also continues after birth and throughout a person’s lifetime.
Activation and Multiplication of Lymphocytes
The specialized B-cell or T-cell clones can respond to antigens and produce an adaptive immune response. For the adaptive immune response to be effective, two events must occur: (1) antigen rec-ognition by lymphocytes and (2) proliferation of the lymphocytes recognizing the antigen.
Antigen Recognition
Lymphocytes have proteins, called antigen receptors, on their surfaces. The antigen receptors on B cells are called B-cell receptors, and those on T cells are called T-cell receptors. Each receptor binds with only a specific antigen.
Each clone consists of lympho-cytes that have identical antigen receptors on their surfaces. When antigens combine with the antigen receptors of a clone, the lym-phocytes in that clone can be activated, and the adaptive immune response begins.
B cells and T cells typically recognize antigens after large mol- ecules have been processed or broken down into smaller fragments Antigen-presenting cells, such as macrophages, present antigens to B and T cells. The antigens are taken into macrophages by phago cytosis and are broken down into smaller antigen fragments. The processed antigen fragments are bound to major histocompatibility complex molecules, transported to the surface of the macrophages, and presented to B cells and T cells (figure 14.10, step 1).
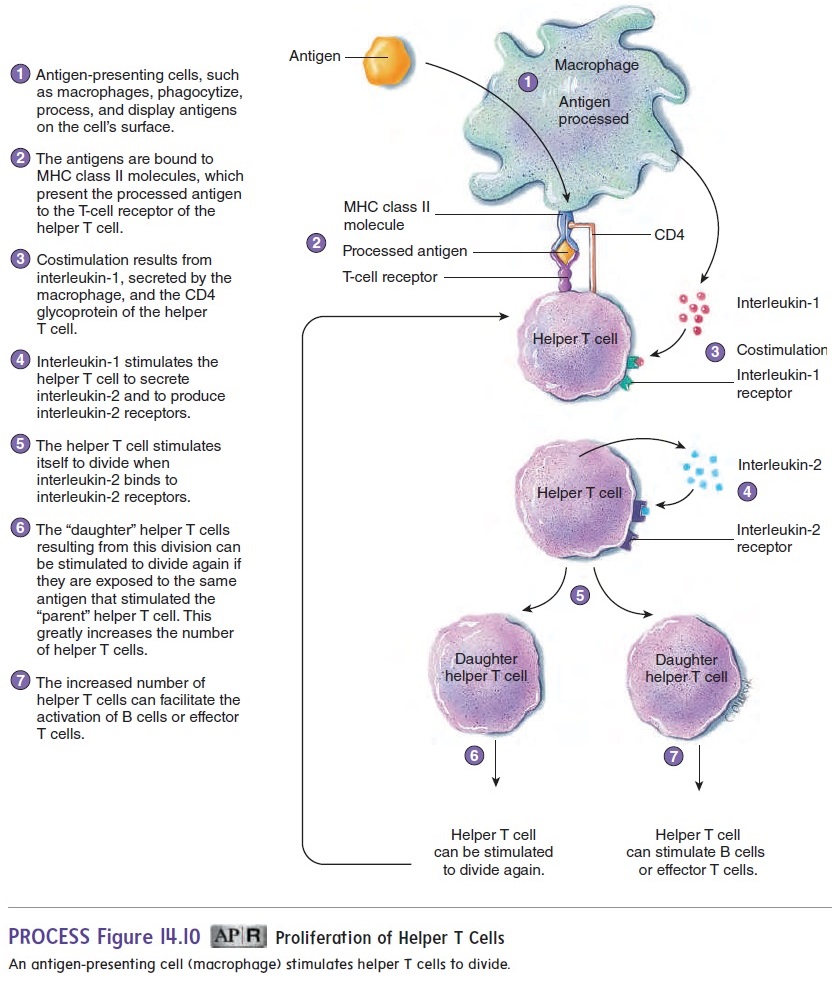
Major histocompatibility complex (MHC) molecules are glycoproteins that have binding sites for antigens. Different MHC molecules have different binding sites—that is, they are specific for certain antigens. It is important not to confuse the MHC molecules with the antigen receptors described above. Though both types of receptors interact with antigens, MHC molecules are a different group of receptors found on the membrane of many types of cells. There are two classes of MHC molecules. MHC class I molecules are found on the membranes of most nucleated cells and MHC class II molecules are found on the membranes of antigen-presenting cells, B lymphocytes, and other defense cells. The MHC molecules function as “serving trays” that hold and present a processed antigen on the outer surface of the cell membrane. The combined MHC molecule and processed antigen can then bind to the antigen recep- tor on a B cell or T cell and stimulate it. For example, figure 14.10, step 2, illustrates how helper T cells are stimulated when combined with MHC class II molecules.
The MHC molecule/antigen combination is usually only the first signal necessary to produce a response from a B cell or T cell.
In many cases, costimulation by a second signal is also required. Costimulation can be achieved by cytokines (sı̄′ tō-kı̄nz), which are proteins or peptides secreted by one cell as a regulator of neighboring cells. For example, interleukin-1 (in-ter-loo′ kin) is a cytokine released by macrophages that can stimulate helper T cells (figure 14.10, step 3).
Lymphocytes have other surface molecules besides MHC molecules that help bind cells together and stimulate a response. For example, helper T cells have a glycoprotein called CD4, which helps connect helper T cells to the macrophage by binding to MHC class II molecules. The CD4 protein is also bound by the virus that causes AIDS (see the Diseases and Disorders table). As a result, the virus preferentially infects helper T cells. Cytotoxic T cells have a glycoprotein called CD8, which helps con-nect cytotoxic T cells to cells displaying MHC class I molecules.
Lymphocyte Proliferation
Before exposure to a particular antigen, the number of helper T cells that can respond to that antigen is too small to produce an effective response against it. After the antigen is processed and presented to a helper T cell by a macrophage, the helper T cell responds by producing interleukin-2 and interleukin-2 receptors(figure 14.10, step 4). Interleukin-2 binds to the receptors and stimulates the helper T cell to divide (figure 14.10, step 5). The “daughter” helper T cells produced by this division can again be presented with the antigen by macrophages and again be stimulated to divide. Thus, the number of helper T cells is greatly increased (figure 14.10, step 6).
It is important for the number of helper T cells to increase because helper T cells are necessary for the activation of most B cells or T cells (figure 14.10, step 7). For example, B cells have receptors that can recognize antigens. Most B cells, however, do not respond to antigens without stimulation from helper T cells. Without functional helper T cells, the immune response of B cells would not be effective to prevent disease.
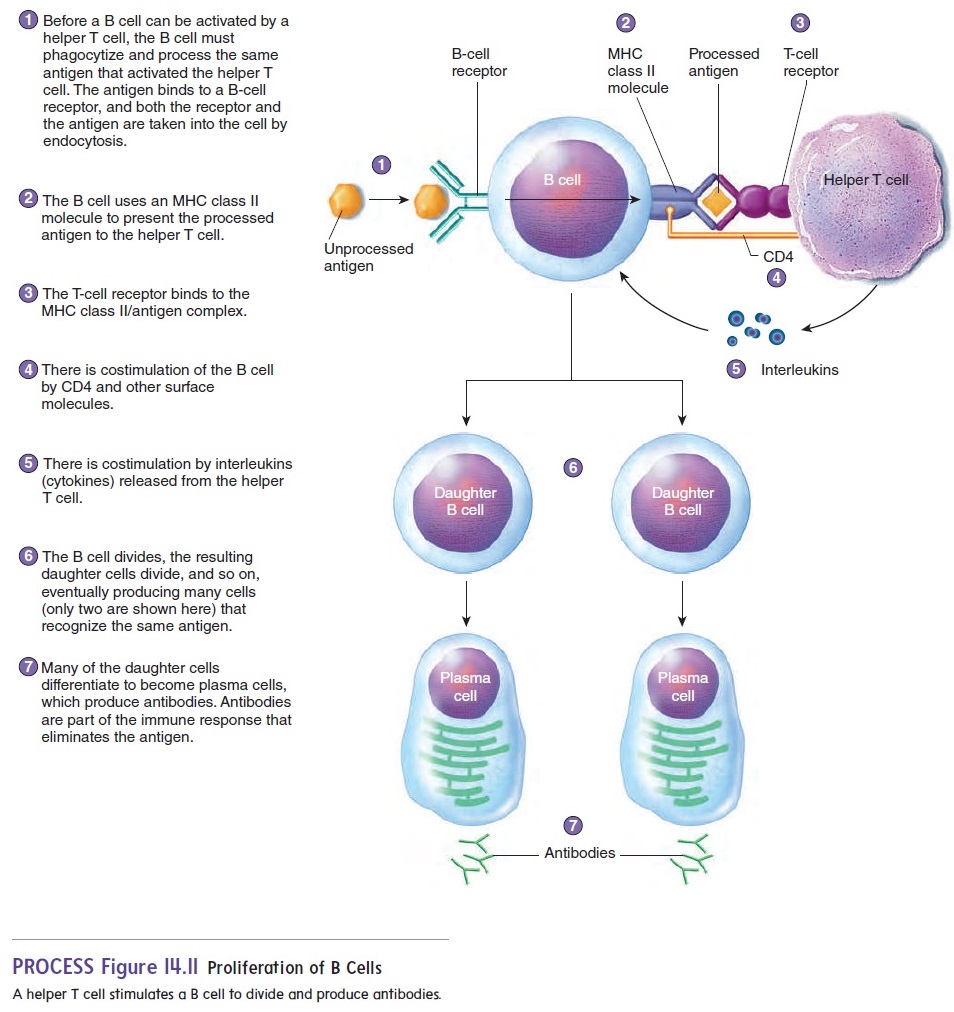
B-cell proliferation begins when a B cell takes in the same kind of antigen that stimulated the helper T cell (figure 14.11, step 1). The antigen is processed by the B cell and presented on the B-cell surface by an MHC class II molecule (figure 14.11, step 2). A helper T cell is stimulated when it binds to the MHC class II/antigen complex (figure 14.11, step 3). There is also costimulation involving CD4 and interleukins (figure 14.11, steps 4 and 5). As a result, the B cell divides into two “daughter” cells (figure 14.11, step 6). These daughter cells may differentiate into plasma cells, which produce antibodies (figure 14.11, step 7).The division process continues, eventually producing many cells capable of producing antibodies and resulting in sufficient anti-bodies to destroy all the antigen.
Antibody-mediated immunity
Exposure of the body to an antigen can lead to the activation of B cells and the production of antibodies. The antibodies bind to the antigens, which can be destroyed through several different mechanisms. Because antibodies are in body fluids, antibody- mediated immunity is effective against extracellular antigens, such as bacteria, viruses (when they are outside cells), and toxins. Antibody-mediated immunity is also involved in certain allergic reactions.
Structure of Antibodies
Antibodies are proteins produced in response to an antigen. They are Y-shaped molecules consisting of four polypeptide chains: two identical heavy chains and two identical light chains (figure 14.12). The end of each “arm” of the antibody is the variable region, the part of the antibody that combines with the antigen. The variable region of a particular antibody can join only with a particular antigen; this is similar to the lock-and-key model of enzymes. The rest of the antibody is the constant region, and it has several functions. For example, the constant region can activate complement, or it can attach the antibody to cells, such as macrophages, basophils, and mast cells.

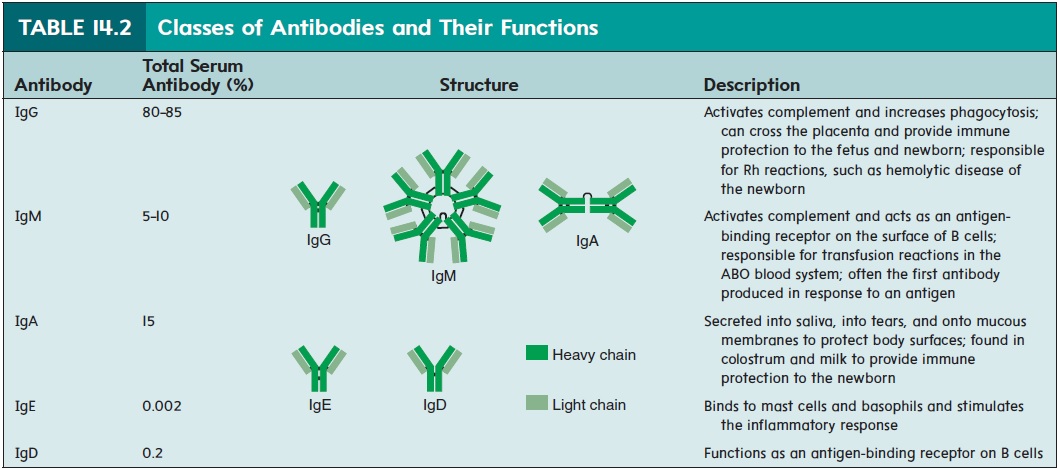
Antibodies make up a large portion of the proteins in plasma. Most plasma proteins can be separated into albumin and alpha, beta, and gamma globulin portions . Antibodies are sometimes called gamma globulins (glob′ ū-linz), because they are found mostly in the gamma globulin part of plasma, or immunoglobulins (Ig), because they are globulin proteins involved in immunity. The five general classes of antibodies are denoted IgG, IgM, IgA, IgE, and IgD (table 14.2).
Effects of Antibodies
Antibodies can affect antigens either directly or indirectly. Direct effects occur when a single antibody binds to an antigen and inactivates the antigen, or when many antigens are bound togetherand are inactivated by many antibodies (figure 14.13a,b). The ability of antibodies to join antigens together is the basis for many clinical tests, such as blood typing, because when enough antigens are bound together, they form visible clumps.
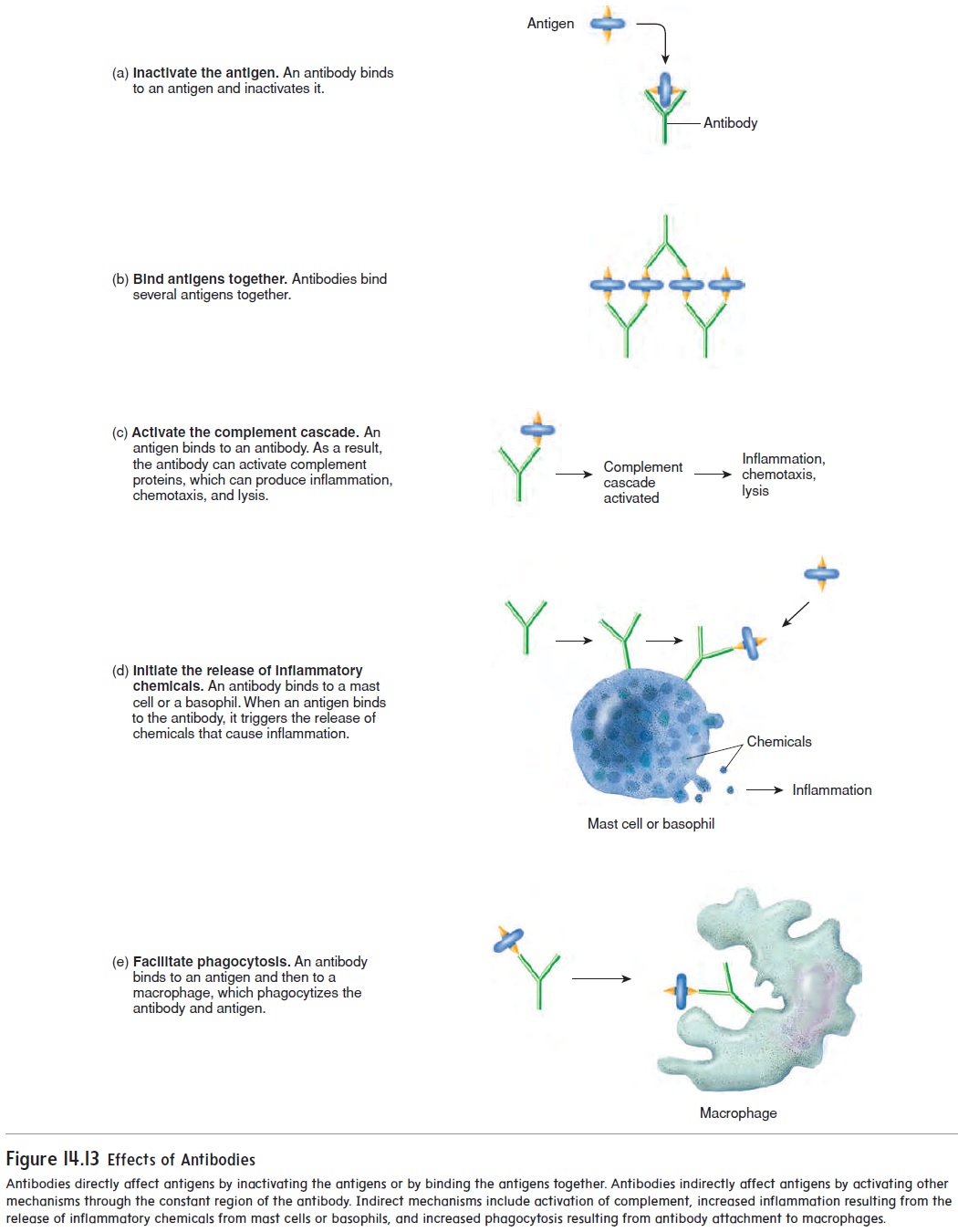
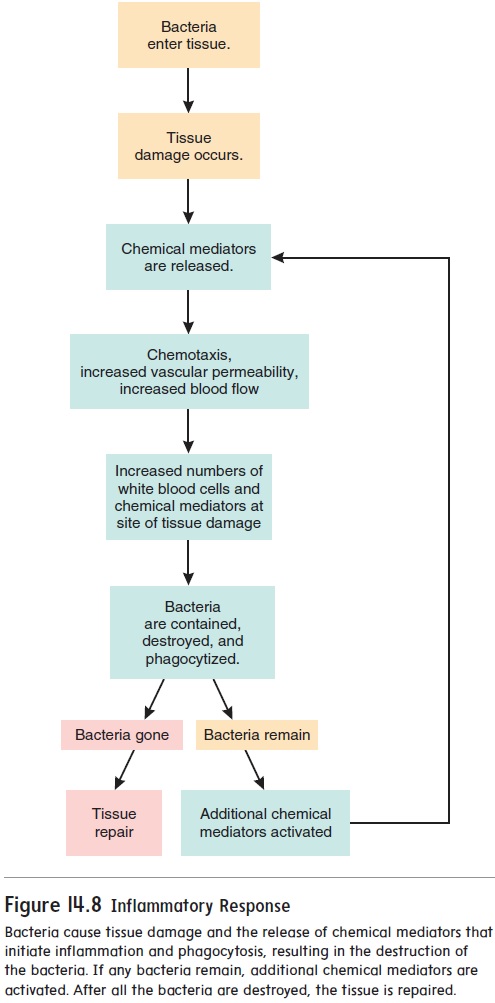
Most of the effectiveness of antibodies results from indirect effects (figure 14.13c–e). After an antibody has attached by its variable region to an antigen, the constant region of the antibody can activate other mechanisms that destroy the antigen. For exam-ple, the constant region of antibodies can activate complement, which stimulates inflammation, attracts white blood cells through chemotaxis, and lyses bacteria. When an antigen combines with the antibody, the constant region triggers the release of inflammatory chemicals from mast cells and basophils. For example, people who have hay fever inhale the antigens (usually plant pollens), which are then absorbed through the respiratory mucous mem-brane. The combination of the antigen with antibodies stimulates mast cells to release inflammatory chemicals, such as histamine. The resulting localized inflammatory response produces swell-ing and excess mucus production in the respiratory tract. Finally, macrophages can attach to the constant region of the antibody and phagocytize both the antibody and the antigen.
Antibody Production
The production of antibodies after the first exposure to an antigen is different from that following a second or subsequent exposure. The primary response results from the first exposure of a B cell to an antigen (figure 14.14, step 1). When the antigen binds to the antigen-binding receptor on the B cell and the B cell has been activated by a helper T cell, the B cell undergoes sev-eral divisions to form plasma cells and memory B cells. Plasma cells produce antibodies. The primary response normally takes 3–14 days to produce enough antibodies to be effective against the antigen. In the meantime, the individual usually develops disease symptoms because the antigen has had time to cause tissue damage.
Memory B cells are responsible for the secondary response, or memory response, which occurs when the immune system is exposed to an antigen against which it has already produced a pri-mary response (figure 14.14, step 2). When exposed to the antigen, the memory B cells quickly divide to form plasma cells, which rapidly produce antibodies. The secondary response provides better protection than the primary response for two reasons: (1) The time required to start producing antibodies is less (hours to a few days), and (2) more plasma cells and antibodies are produced. As a con-sequence, the antigen is quickly destroyed, no disease symptoms develop, and the person is immune.
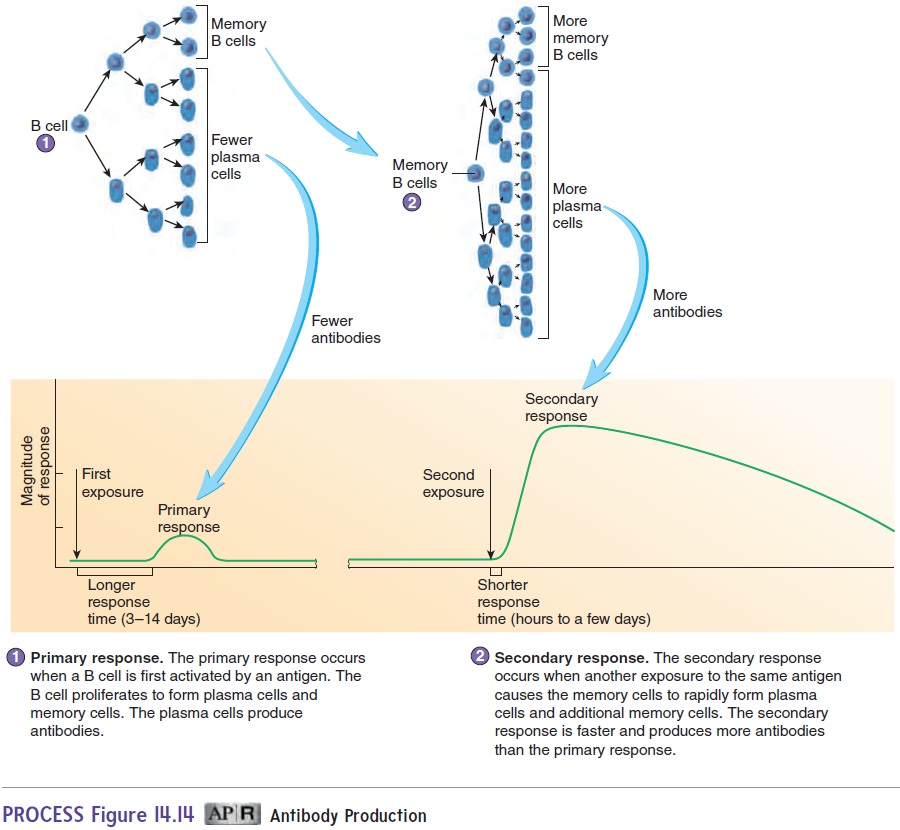
The secondary response also includes the formation of new memory cells, which provide protection against additional expo-sures to a specific antigen. Memory cells are the basis of adaptive immunity. After destruction of the antigen, plasma cells die, the antibodies they released are degraded, and antibody levels decline to the point where they can no longer provide adequate protection. However, memory cells persist for many years—for life, in some cases. If memory cell production is not stimulated, or if the memory cells produced are short-lived, it is possible to have repeated infec-tions of the same disease. For example, the same cold virus can cause the common cold more than once in the same person.
Cell-Mediated Immunity
Cell-mediated immunity is a function of cytotoxic T cells and is most effective against microorganisms that live inside body cells. Viruses and some bacteria are examples of intracellular micro-organisms. Cell-mediated immunity is also involved with some allergic reactions, the control of tumors, and graft rejection.
Cell-mediated immunity is essential for fighting viral infections. When viruses infect cells, they direct the cells to make new viruses, which are then released to infect other cells. Thus, cells are turned into virus-manufacturing plants. While inside the cell, viruses have a safe haven from antibody-mediated immunity because antibodies cannot cross the cell membrane. Cell-mediated immunity fights viral infections by destroying virally infected cells. When viruses infect cells, some viral proteins are broken down and become pro-cessed antigens that are combined with MHC class I molecules and displayed on the surface of the infected cell (figure 14.15, step 1).
Cytotoxic T cells can distinguish between virally infected cells and noninfected cells because the T-cell receptor can bind to the MHC class I/viral antigen complex, which is not present on uninfected cells.
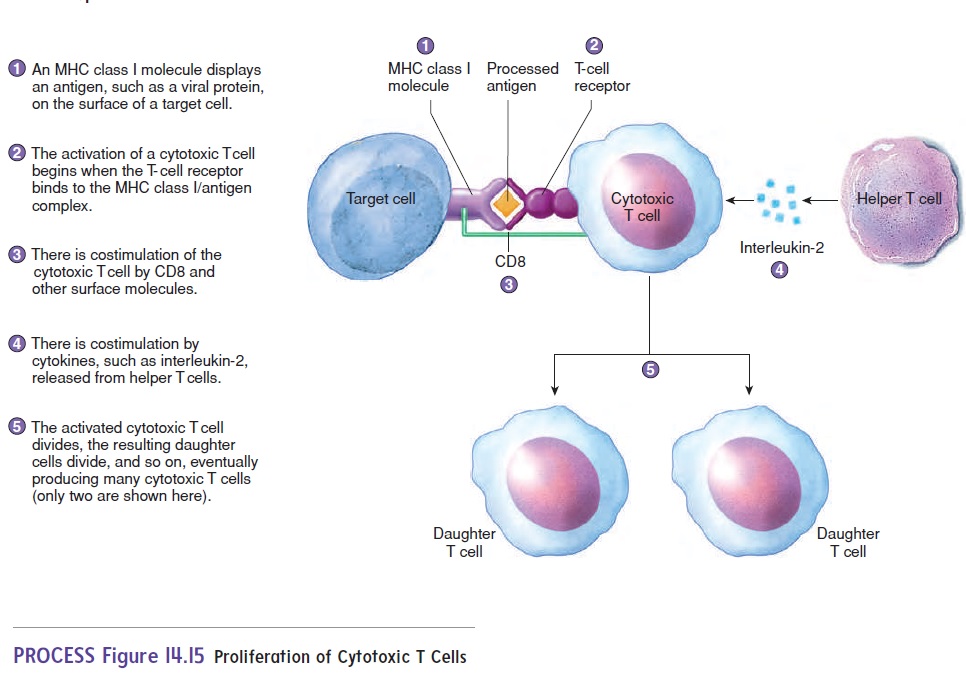
The T-cell receptor binding with the MHC class I/antigen com-plex is a signal for activating cytotoxic T cells (figure 14.15, step 2). Costimulation by other surface molecules, such as CD8, also occurs (figure 14.15, step 3). Helper T cells provide costimulation by releasing cytokines, such as interleukin-2, which stimulate activa-tion and cell division of cytotoxic T cells (figure 14.15, step 4).
Increasing the number of “daughter” helper T cells results in greater stimulation of cytotoxic T cells. In cell-mediated responses, helper T cells are activated and stimulated to divide in the same fashion as in antibody-mediated responses (see figure 14.10).
After cytotoxic T cells are activated by an antigen on the sur-face of a target cell, they undergo a series of divisions to produce additional cytotoxic T cells and memory T cells (figure 14.16). The cytotoxic T cells are responsible for the cell-mediated immune response, and the memory T cells provide a secondary response and long-lasting immunity in the same fashion as memory B cells.
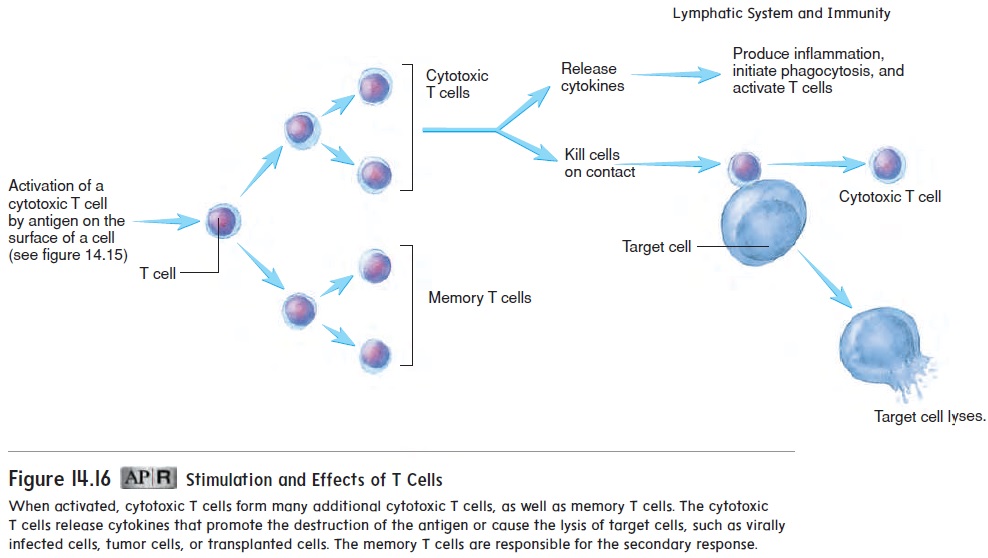
Cytotoxic T cells have two main effects:
1. They release cytokines that activate additional components of the immune system. For example, some cytokines attract innate immune cells, especially macrophages. These cells are then responsible for phagocytosis of the antigen and the production of an inflammatory response. Cytokines also activate additional cytotoxic T cells, which increases the effectiveness of the cell-mediated response.
2. Cytotoxic T cells can come in contact with other cells and kill them. Virally infected cells have viral antigens, tumor cells have tumor antigens, and tissue transplants have foreign antigens that can stimulate cytotoxic T-cell activity. The cytotoxic T cells bind to the antigens on the surfaces of these cells and cause the cells to lyse.
Related Topics