Chapter: Plant Biochemistry: Phenylpropanoids comprise a multitude of plant secondary metabolites and cell wall components
The synthesis of flavonoids and stilbenes requires a second aromatic ring derived from acetate residues
The synthesis of flavonoids and stilbenes requires a second aromatic ring derived from acetate residues
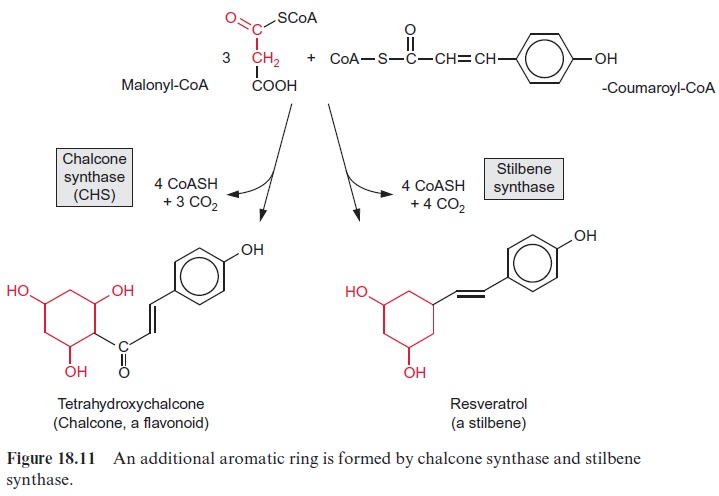
Probably the largest group of phenylpropanoids is that of the flavonoids, in which a second aromatic ring is linked to the 9’ -C atom of the phenyl-propanoid moiety. A precursor for the synthesis of flavonoids is chalcone (Fig. 18.11), synthesized by chalcone synthase (CHS) from p-coumaryl-CoA and three molecules of malonyl-CoA.This reaction is also called the malonate pathway. The release of three CO2 molecules and four CoA molecules makes chalcone synthesis an irreversible process. In the overall reaction, the new aromatic ring is formed from three acetate residues. Since CHS represents the first step of flavonoid biosynthesis, this enzyme has been thoroughly investigated. In some plants, one or two different isoforms of the enzyme have been found, while in others there are up to nine. CHS is the most abundant enzyme protein of phenylpropanoid metabolism in plant cells, probably because this enzyme has only a low catalytic activity. As in the case of phenylalanine ammonia lyase , the de novo synthesis of CHS is subject to multiple controls of gene expression by inter-nal and external factors, including elicitors.
Some stilbenes are very potent natural fungicides
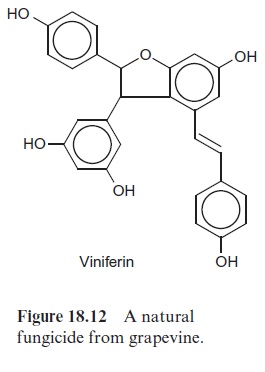
Some plants, including pine, grapevine and peanuts, possess a stilbene syn-thase activity, by which p-coumaroyl-CoA reacts with three molecules of malonyl CoA. In contrast to CHS, the 9’ -C atom of the phenylpropane is released as CO2 (Fig. 18.11). Resveratrol, synthesized by this process, is a phytoalexin belonging to the stilbene group. A number of very potent plant fungicides are stilbenes, including viniferin (Fig. 18.12), which is contained in grapevine. The elucidation of stilbene synthesis has opened new possibil-ities to combat fungal infections. A gene from grapevine for the formation of resveratrol has been expressed by genetic engineering in tobacco, and the resultant transgenic tobacco plants were resistant to the pathogenic fungus Botrytis cinerea.
Related Topics