Chapter: Plant Biochemistry: The Calvin cycle catalyzes photosynthetic CO2 assimilation
The reduction of 3-phosphoglycerate yields triose phosphate
The reduction of 3-phosphoglycerate yields triose phosphate
For the synthesis of dihydroxyacetone phosphate the carboxylation prod uct 3-phosphoglycerate is phosphorylated to 1,3-bisphosphoglycerate by the enzyme phosphoglycerate kinase. In this reaction, with the consumption of ATP, a mixed anhydride is formed between the new phosphate residue and the carboxyl group (Fig. 6.9). As the free energy for the hydrolysis of this anhydride is similarly high to that of the phosphate anhydride in ATP, the phosphoglycerate kinase reaction is reversible. An isoenzyme of the chloroplast phosphoglycerate kinase is also involved in the glycolytic pathway proceeding in the cytosol, where it catalyzes the formation of ATP from ADP and 1,3-bisphosphoglycerate .
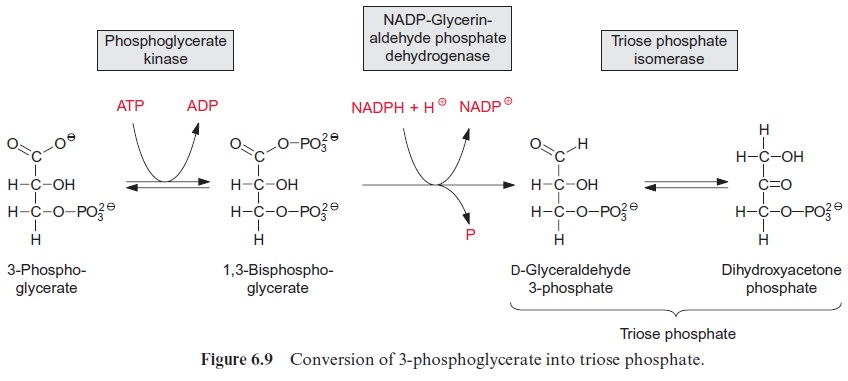
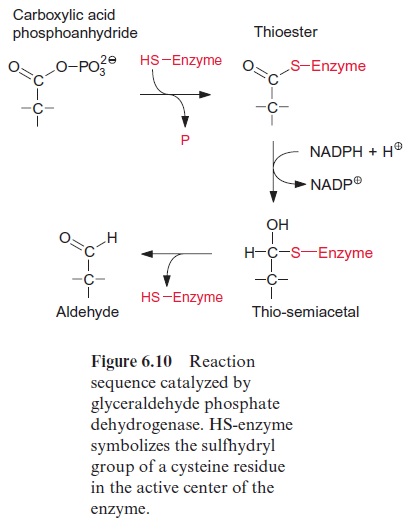
The reduction of 1,3-bisphosphoglycerate to D-glyceraldehyde 3-phos phate is catalyzed by the enzyme glyceraldehyde phosphate dehydrogenase (Fig. 6.9). The carboxylic acid phosphoanhydride reacts with an SH-group of a cysteine residue in the active center of the enzyme to form a thioester intermediate with the release of the phosphate group (Fig. 6.10). The free energy for the hydrolysis of the thioester so formed is similarly high to that of the anhydride (“energy-rich bond”). When a thioester is reduced, a thio-semiacetal is formed which has low free energy.
Through the catalysis of phosphoglycerate kinase and glyceraldehyde phosphate dehydrogenase, the large difference in redox potentials between the carboxylate and the aldehyde in the course of the reduction of 3-phos phoglycerate to glyceraldehyde phosphate is overcome by the consumption of ATP. It is therefore a reversible reaction. A glyceraldehyde phosphate dehydrogenase in the cytosol catalyzes the conversion of glyceraldehyde phosphate to 1,3-bisphosphoglycerate as part of the glycolytic pathway . In contrast to the cytosolic enzyme, which catalyzes mainly the oxidation of glyceraldehyde phosphate using NAD+ as hydrogen acceptor, the chloroplast enzyme uses NADPH as a hydrogen donor.
This is an example of the different roles that the NADH/NAD+ and NADPH/NADP+ systems play in the metabolism of eukaryotic cells. Whereas the NADH system is specialized in collecting reducing equivalents to be oxidized for the synthesis of ATP, the NADPH system mainly gathers reducing equivalents to be donated to synthetic processes. Figuratively speaking, the NADH system has been compared with a hydrogen low pressure line through which reducing equivalents are pumped off for oxi dation to generate energy, while the NADPH system is a hydrogen high pressure line through which reducing equivalents are pressed into synthe sis processes. Usually the reduced/oxidized ratio is about 100 times higher for the NADPH system than for the NADH system. The relatively high degree of reduction of the NADPH system in chloroplasts (about 50–60% reduced) allows the very efficient reduction of 1,3-bisphosphoglycerate to glyceraldehyde-3-phosphate.
Triose phosphate isomerase catalyzes the isomerization of glyceralde hyde phosphate to dihydroxyacetone phosphate. This conversion of an aldose into a ketose proceeds via a 1,2-enediol as intermediate and is basi cally similar to the reaction catalyzed by ribose phosphate isomerase. The equilibrium of the reaction lies towards the ketone. Triose phosphates, as a collective term, comprise about 96% dihydroxyacetone phosphate and only 4% glyceraldehyde phosphate.
Related Topics