Chapter: Biochemical Pharmacology : The ionic basis of cell excitation
The physics of membrane potentials
The physics of membrane
potentials
Both the resting and the
action potential are diffusion potentials. A diffusion potential arises at a
membrane if
1. the membrane is selectively permeable for one
or a few ion species,
2. ls exist will therefore have a say in
determining the membrane potential. Furthermore, as ion channels open and
close, the changing permeabilities can shift the weight from one ion to the
other. The most important example is the transient opening of sodium channels,
which according to the Goldman equation will cause the membrane potential to
shift toward the equilibrium potential.
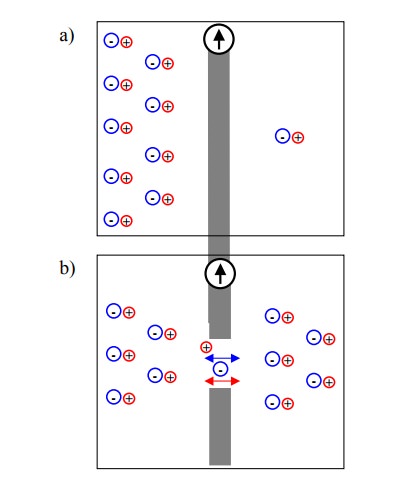
Figure 4.2a shows a membrane
that has an ion gradient across it but is entirely impermeable; this will
result in no membrane potential, as long as the numbers of anions and cations
are the same within each adjoining compartment. In Figure 4.2b, the membrane
has been rendered perme-able by a large, non-selective hole; both anions and
cations are free to equilibrate across the membrane – again, there will be no
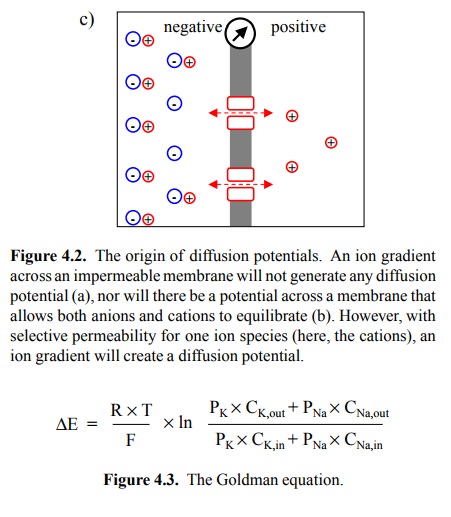
membrane potential. In Figure 4.2c, the membrane contains channels that are
selectively permeable to the cations. Diffusion of cations down their
concentration gradient will create an imbalance of charge that in turn gives
rise to a potential (a voltage gradient) across the membrane.
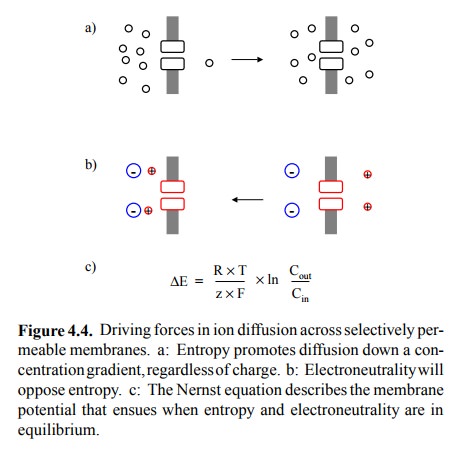
The migrating cations depicted in Figure 4.2c
are subject to two driving forces that act in opposite directions: The
concentration gradient will tend to drive any permeable species, regardless of
its charge, across the membrane, until the concentrations are identical on both
sides (Figure 4.4a); this is simply the maximization of entropy. Conversely,
with progressing diffusion, the membrane potential will rise ever higher and
tend to pull the cations back to the left side (Figure 4.4b). At some point,
the two forces will be equally strong, and no further net ion movement will
occur. This point is the equilibrium
potential.
The equilibrium potential can be calculated
from the Nernst equation, which is simply an application of the Gibbs equa-tion
to ion gradients (Figure 4.4c)2.
You will note that most terms
in that equation are con-stants (the temperature is virtually constant at 37 °C = 310 K, too), so that the
potential is essentially a function of the ion concentration gradient alone.
However, this simple re-lationship will hold only when there is only one
diffusible ion species. In a cell, we have several ion species, and fi-nite
permeabilities for several of them. The two dominant cations, Na+
and K+, have roughly opposite distributions across the plasma
membrane. Application of the Nernst equation to Na+ and K+
would yield membrane potentials of +60 mV and -90 mV, respectively3.
Yet, the actual resting membrane potential is not identical to either of these
values (which it would if the membrane were selectively perme-able to either K+
or Na+ only). Clearly, what we need is a means to determine the
membrane potential if we have mul-tiple permeable ion species. This is provided
by the Gold-man equation (given in Figure 4.3 in its special case for K+
and Na+). When comparing it with the Nernst equation, you see that
the Goldman equation introduces one additional parameter P (for permeability),
which has a specific value for each ion present.
Thus, the contribution of
each ion to the overall membrane potential depends not only on its
concentration but it is weighted for the permeability of the membrane for that
ion. Since the concentrations and gradients for K+ and Na+
are of similar magnitude, we may infer that the membrane permeability must be
larger for potassium than for sodium (PK > PNa), since the
actual membrane potential is much closer to the K+ than to the Na+
equilibrium potential.
Now what's the molecular
basis of different permeabili-ties for different ions? This is where the
channels come in. Without a specific channel, no ion can effectively cross the
membrane, so its permeability will be very small; only ions for which specific
channels exist will therefore have a say in determining the membrane potential.
Furthermore, as ion channels open and close, the changing permeabilities can
shift the weight from one ion to the other. The most impor-tant example is the
transient opening of sodium channels, which according to the Goldman equation
will cause the membrane potential to shift toward the sodium equilibrium
potential.
Related Topics