Chapter: Organic Chemistry: Alcohols, phenols, and thiols
Properties of alcohols and phenols
PROPERTIES OF ALCOHOLS AND PHENOLS
Key Notes
Alcohols
The
carbon and oxygen atoms of the alcohol group are sp3 hybridized such that the C–O–H bond angle is
approximately 109 . Hydrogen bonding means that alcohols have higher boiling
points than comparable alkanes. Alcohols of low molecular weight are soluble in
water and can act as weak acids and weak bases. Alcohols are polar. The oxygen
atom is a nucleophilic center while the neighboring carbon and hydrogen are
weak electrophilic centers. Alcohols will not react with nucleophiles, but will
react with strong bases in an acid–base reaction to form an alkoxide ion. An
alcohol’s C–O bond can be split if the hydroxyl group is converted into a
better leaving group.
Phenols
Phenols
have an OH group directly linked to an aromatic ring. The oxygen is sp3 hybridized and the aryl
carbon is sp2 hybridized.
Phenols are polar compounds which are capable of intermolecular hydrogen
bonding such that phenols have higher boiling points than nonphenolic aromatic
struc-tures of comparable molecular weight. Hydrogen bonding also permits
moderate water solubility and phenols act as weak acids in aqueous solu-tion.
Phenols are stronger acids than alcohols but weaker acids than carboxylic
acids. They are soluble as their phenoxide salts in sodium hydroxide solution,
but insoluble in sodium hydrogen carbonate solution.
Spectroscopic analysis of alcohols and phenols
Alcohols
and phenols can be identified by the presence of an O–H stretch-ing absorption
in the IR spectrum as well as a D2O exchangeable OH signal in the 1H
nmr spectrum. Further evidence can be obtained from the IR spec-trum if
absorptions due to O–H bending and C–O stretching are identifi-able. In nmr
spectra, the chemical shifts of neighboring groups give indirect evidence of an
OH group. The molecular ion is often absent from the mass spectrum due to rapid
dehydration.
Alcohols
The alcohol functional group (R3C–OH)
has the same geometry as water, with a C–O–H bond angle of approximately 109°. Both the carbon and the oxygen are sp3 hybridized. The presence
of the O–H group means that intermolecular hydrogen bonding is possible which
accounts for the higher boiling points of alcohols compared with alkanes of
similar molecular weight. Hydrogen bonding also means that alcohols are more
soluble in protic solvents than alkanes of similar molecular weight. In fact,
the smaller alcohols (methanol, ethanol, propanol, and tert-butanol) are completely miscible in water. With larger
alcohols, thehydrophobic character of the bigger alkyl chain takes precedence
over the polar alcohol group and so larger alcohols are insoluble in water.
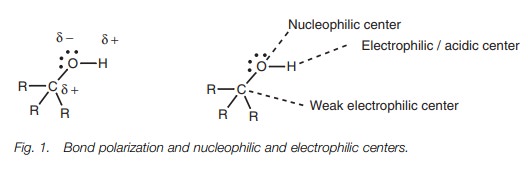
The O–H and C–O bonds are both polarized due to
the electronegative oxygen, such that oxygen is slightly negative and the
carbon and hydrogen atoms are slightly positive. This means that the oxygen
serves as a nucleophilic center while the hydrogen and the carbon atoms serve
as weak electrophilic centers.
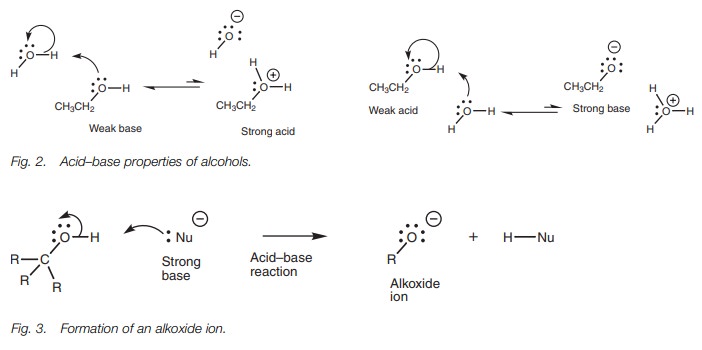
Due to the presence of the nucleophilic oxygen
and electrophilic proton, alco-hols can act both as weak acids and as weak
bases when dissolved in water (Fig.2).
However, the equilibria in both cases is virtually completely weighted to
theunionized form.
Alcohols will commonly react with stronger electrophiles than water. However, they are less likely to react with nucleophiles unless the latter are also strong bases, in which case the acidic proton is abstracted to form an alkoxide ion (RO ;Fig. 3). Alkoxide ions are extremely useful reagents in organic synthesis. However,
they cannot be used if water is the solvent
since the alkoxide ion would act as a base and abstract a proton from water to
regenerate the alcohol. Therefore, an alcohol would have to be used as solvent
instead of water.
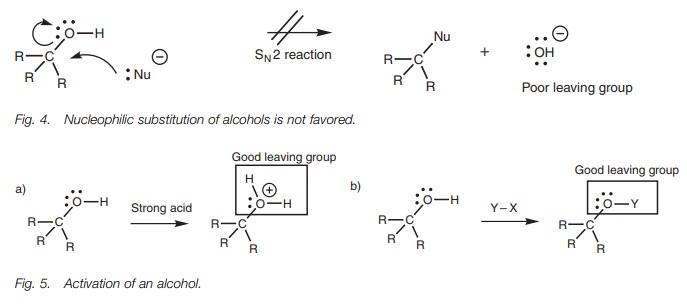
Nucleophiles which are also strong bases react with the electrophilic hydrogen of an alcohol rather than the electrophilic carbon. Nucleophilic attack at carbon would require the loss of a hydroxide ion in a nucleophilic substitution reaction. However, this is not favored since the hydroxide ion is a strong base and a poor leaving group (Fig. 4). Nevertheless, reactions which involve the cleav-age of an alcohol’s C–O bond are possible if the alcohol is first ‘activated’ such that the hydroxyl group is converted into a better leaving group. One method is to react the alcohol under acidic conditions such that the hydroxyl group is protonated before the nucleophile makes its attack (Fig. 5a). Cleavage of the C–O bond would then be more likely since the leaving group would be a neutral water molecule, which is a much better leaving group. Alternatively, the alcohol can be treated with an electrophilic reagent to convert the OH group into a different group (OY) which can then act as a better leaving group (Fig. 5b). In both cases, the alcohol must first act as a nucleophile, with the oxygen atom act-ing as the nucleophilic center. The intermediate formed can then react more read-ily as an electrophile at the carbon center.
Phenols
Phenols are compounds which have an OH group
directly attached to an aromatic ring. Therefore, the oxygen is sp3 hybridized and the aryl
carbon is sp2 hybridized.
Although phenols share some characteristics with alcohols, they have distinct
properties and reactions which set them apart from that functional group.
Phenols can take part in intermolecular
hydrogen bonding, which means that they have a moderate water solubility and
have higher boiling points than aro-matic compounds lacking the phenolic group.
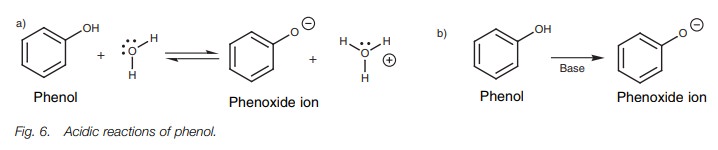
Phenols are weakly acidic, and in aqueous solution an equilibrium exists
between the phenol and the phenoxide ion (Fig.
6a). On treatment with a base, the phenol is fully converted to the
phenoxide ion (Fig. 6b).
The phenoxide ion is stabilized by resonance and delocalization of the negative charge into the ring, which means that phenoxide ions are weaker bases than alkoxide ions. This in turn means that phenols are more acidic than alcohols, but less acidic than carboxylic acids. The pKa values of most phenols is in the order of 11, compared to 18 for alcohols and 4.74 for acetic acid.
This means that phenols can be ionized with weaker
bases than those required to ionize alcohols, but require stronger bases than
those required to ionize carboxylic acids. For example, phenols are ionized by
sodium hydroxide but not by the weaker base sodium hydrogen carbonate. Alcohols
being less acidic are not ionized by either base whereas carboxylic acids are
ionized by both sodium hydroxide and sodium hydrogen carbonate solutions.
These acid–base reactions permit a simple way
of distinguishing between most carboxylic acids, phenols, and alcohols. Since
the salts formed from the acid–base reaction are water soluble, compounds
containing these functional groups can be distinguished by
testing their solubilities
in sodium hydrogen
carbonate and sodium hydroxide
solutions. This solubility test is not valid for low molecular weight
structures such as methanol or ethanol since these are water soluble and
dissolve in basic solution because of their water solubility rather than their
ability to form salts.
Spectroscopic analysis of alcohols and phenols
The IR spectra of alcohols and phenols give
characteristic broad O–H stretching absorptions in the region 3600–3200 cm−1. These absorptions are broader than N–H
absorptions but are not as broad as the O–H absorption of a carboxylic acid.
The exact position of the absorption depends on the extent of hydrogen bonding
in the sample. The more H-bonding which is present, the broader the absorption
and the lower the wavenumber.An absorption due to O–H bending may be visible in
the region 1410–1260 cm−1, but this is in the fingerprint region and can
easily be confused with other absorptions. Absorptions due to C–O stretching
also occur in the fingerprint region, but can sometimes be distinguished since
they tend to be stronger than surrounding absorptions. They occur in the
regions 1075–1000 cm−1
for primary alcohols,
1125–1100 cm−1
for secondary alcohols, 1210–1100 cm−1 for
tertiary alcohols, and 1260–1140 cm−1 for phenols.
The OH proton is visible in the 1H nmr spectra
of alcohols and phenols, nor- mally as a broad signal in the region 0.5–4.5 ppm
for alcohols and 4.5–10 ppm for phenols. The signal is lost if the sample is
shaken with deuterated water.
The presence of an alcohol can sometimes be
indicated indirectly by the chem- ical shifts of neighboring groups. For
example, a methylene unit next to OH appears at 3.6 ppm in the 1H spectrum.
Carbon atoms next to OH show signals in the range 50–80 ppm in the 13C
spectrum.
The mass spectra of alcohols often lack the
molecular ion since dehydration can occur rapidly to give a fragmentation ion
18 mass units less than the parent ion. Fragmentation also occurs by
α-cleavage, i.e. cleavage, next to the carbon, which is bonded to the OH.
Related Topics