Chapter: Biochemistry: Protein Metabolism
Metabolism of Proteins
Metabolism of Proteins
The ingested proteins are metabolised to amino
acids by peptide bond cleaving enzymes known as proteinases.
1. General reactions of amino acids
The general reactions of amino acids include
deamination, transamination and decarboxylation. The reactions of deamination
and transamination bring about the formation of keto acids which can undergo a
further series of changes. Inter-conversion between keto acids and amino acids
results in the synthesis of many nutritionally non essential amino acids. These
provide for the synthesis of protein and important non-protein nitrogenous
materials. During protein synthesis the amino acids are absorbed from the
blood, as the liver does not store them.
2. Catabolism of amino acids
Although each amino acid follows its own
specific metabolic pathway, a few general reactions are found to be common in
the catabolism of nearly all the amino acids. Most of the amino acids are
converted to a-keto acids by the removal of nitrogen in the form of ammonia
which is quickly transformed into urea or it gets incorporated into some other
amino acids.
Oxidative deamination
Deamination means removal of the amino groups
from amino acids. This is the mechanism where in the amino acids lose two
hydrogen atoms (dehydrogenation) to form keto acids and ammonia.
Oxidative deamination is accompanied by
oxidation and is catalysed by specific amino acid oxidases or more
appropriately, dehydrogenases present in liver and kidneys. The process of
oxidative deamination takes place in two steps.
The first step is oxidation (dehydrogenation) of
amino acid resulting in the formation of imino acid. The imino acid then
undergoes the second step, namely hydrolysis which results in a keto acid and
ammonia.
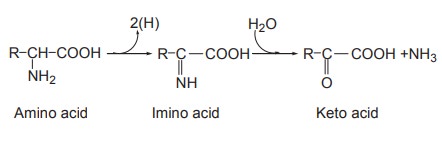
The first reaction is catalyzed by amino acid
oxidase (also called dehydrogenase) and the coenzyme FAD or FMN takes up the
hydrogen. There are two types of amino acid oxidases depending upon the
substrate on which they act, namely,
·
L-amino
acid oxidases which act on L-amino acids (FMN acts as coenzyme).
·
D-amino
acid oxidases which act on D-amino acids (FAD acts as coenzyme).
FMN occurs only in the liver and kidney and FAD
occurs in all animal tissues. The major site of oxidative deamination is liver
but kidney and other tissues also have a role.
The oxidative deamination of L-glutamic acid is
an exceptional case where the deamination needs not only the zinc-containing
enzyme L-glutamic acid dehydrogenase but also NAD+ or NADP+ as coenzymes.
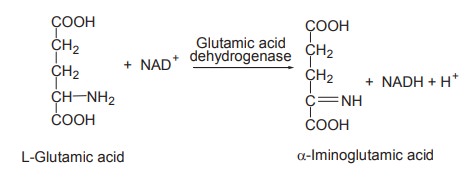
NADH gets oxidized to NAD+ as it passes through
the electron transport chain.
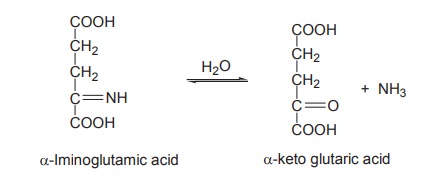
As the above reaction is reversible it occurs
during both amino acid catabolism and biosynthesis.
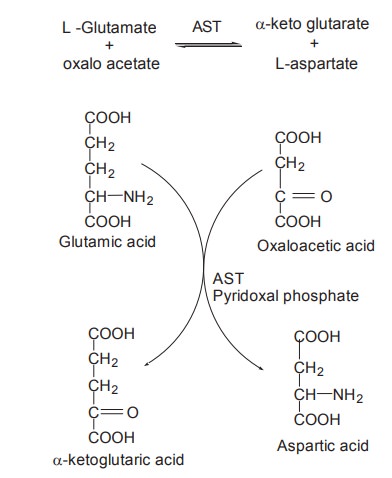
Transamination
The process of transfer of an amino group from
an amino acid to an a-keto acid, resulting in the formation of a new amino acid
and keto acid is known as transamination. In other words, it is deamination of
an amino acid, coupled with amination of a keto acid.
Transamination is catalyzed by transaminases or
aminotransferases with pyridoxal phosphate functioning as coenzyme. There are
two active transaminases in tissues, catalyzing interconversions. They are
·
Aspartate
aminotransferase (AST) is also known as Glutamate
-oxalo acetate
transaminase (GOT)
·
Alanine
aminotransferase (ALT) is also known as Glutamate
-pyruvate
transaminase (GPT)
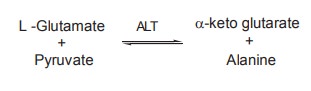
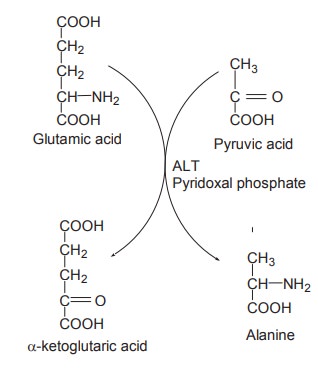
AST
ALT
It catalyses the transfer of NH2
group from glutamate to pyruvate, resulting in the formation of a-ketoglutaric
acid and alanine.
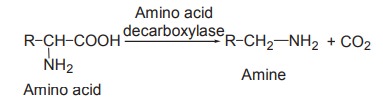
Decarboxylation
This refers to the removal of CO2
from the carboxyl group of amino acids. The removal of CO2 needs the
catalytic action of enzymes decarboxylases and the pyridoxal phosphate
coenzyme. The enzymes act on amino acids resulting in the formation of the
corresponding amines with the liberation of CO2.
There are several amino acid decarboxylases
found in various tissues such as liver, kidney, intestine, spleen, lung and
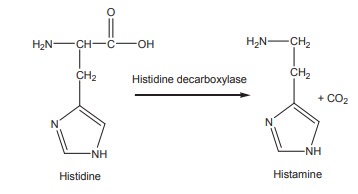
brain. They convert the amino acids into the respective amines and liberate CO2.
For example, histidine is converted to histamine by the action of histidine
decarboxylase.
The amino acid tryptophan is converted to
tryptamine, tyrosine to tyramine, etc. Such amines are called biogenic amines
which are physiologically important.
Transmethylation
The transfer of methyl group from one compound
to another is called transmethylation and the enzymes involved in the transfer
are known as transmethylases.
Transfer of methyl group usually involves
methionine (amino acid containing methyl group). By this process various
important, physiologically active compounds such as epinephrine, creatine,
thymine and choline are synthesised in the body. Detoxification of certain
toxic substances are also carried out by this process (eg. nicotinic acid is
detoxified by methionine into a nontoxic methyl derivative namely N-methyl
nicotinamide).
Methionine is a principal methyl donor. It has
to be activated by ATP which requires a methionine activation enzyme of
liver,known as methionine adenosyl transferase. By the action of this enzyme,
methionine is converted to active methionine.
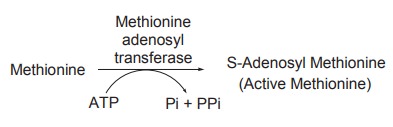
The active methionine thus formed is known as
S-adenosyl methionine and in the activation reaction, ATP transfers its
adenosine moiety to methionine and loses three molecules of phosphate, one as
orthophosphate(Pi) and two as pyrophosphate (PPi).
Active Methionine + Norepinephrine ------------> Epinephrine
Active Methionine +
Nictoinamide ------------> N-methyl nicotinamide
Active Methionine +
Uracil
------------> Thymine
Active Methionine +
Guanido acetate
------------> Creatine
(Methyl group donor) (Methyl group acceptor)
Active methionine contains S-methyl bond which
is a high energy bond and hence methyl group is liable and can be easily
transferred to a methyl group acceptor.
Catabolism of the carbon skeleton of amino acids
The carbon skeletons left behind after
deamination are identified as a-keto acids. They may take any one of the following
pathways.
·
Synthesis of amino acids
They may get reductively aminated by reversal of
transdeamination or undergo transamination to form once again the original
amino acids.
·
Glucogenic pathway
The keto acids of some amino acids may get
converted to the intermediates of carbohydrate metabolism such as a-keto
glutarate, oxaloacetate, pyruvate, fumarate and succinyl CoA and hence could be
converted to glucose and glycogen and these amino acids are said to by
glucogenic amino acids.
The pathways of three important glucogenic amino
acids are shown below. Though the routes vary with each amino acid, they all
converge at the stage of pyruvic acid.
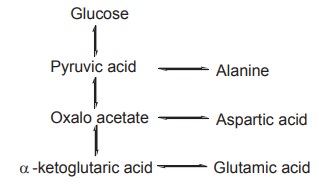
Glucogenic amino acids constitute more than 50%
of the amino acids, derived from animal protein. The process of conversion of
the keto acids of glucogenic amino acids to carbohydrate metabolites is known
as gluconeogenesis.
·
Ketogenic pathway
The keto acids formed from the deamination of
certain amino acids are closely related to fats rather than carbohydrates. They
metabolise to form acetyl CoA or acetoacetyl CoA or acetoacetate (ketone
bodies) which are the intermediates of fatty acid metabolism and not glucose
and these amino acid are said to be ketogenic amino acids.
Ketogenic amino acids constitute only a minority
and follow specialised and complex pathways. Examples are leucine, isoleucine,
phenyl alanine and tyrosine. Among these, leucine is purely ketogenic, whereas
the other three amino acids are both ketogenic and glucogenic.
Related Topics