Chapter: Medical Physiology: Insulin, Glucagon, and Diabetes Mellitus
Insulin and Its Metabolic Effects
Insulin and Its Metabolic Effects
Insulin was first isolated from the pancreas in 1922 by Banting and Best, and almost overnight the outlook for the severely diabetic patient changed from one of rapid decline and death to that of a nearly normal person. Historically, insulin has been associated with “blood sugar,” and true enough, insulin has profound effects on carbohydrate metabolism. Yet it is abnormalities of fat metabolism, causing such conditions as acidosis and arteriosclerosis, that are the usual causes of death in diabetic patients. Also, in patients with prolonged diabetes, dimin-ished ability to synthesize proteins leads to wasting of the tissues as well as many cellular functional disorders. Therefore, it is clear that insulin affects fat and protein metabolism almost as much as it does carbohydrate metabolism.
Insulin Is a Hormone Associated with Energy Abundance
As we discuss insulin in the next few pages, it will become apparent that insulin secretion is associated with energy abundance. That is, when there is great abundance of energy-giving foods in the diet, espe-cially excess amounts of carbohydrates, insulin is secreted in great quantity. In turn, the insulin plays an important role in storing the excess energy. In the case of excess carbohydrates, it causes them to be stored as glycogen mainly in the liver and muscles. Also, all the excess carbohydrates that cannot be stored as glyco-gen are converted under the stimulus of insulin into fats and stored in the adipose tissue. In the case of pro-teins, insulin has a direct effect in promoting amino acid uptake by cells and conversion of these amino acids into protein. In addition, it inhibits the break-down of the proteins that are already in the cells.
Insulin Chemistry and Synthesis
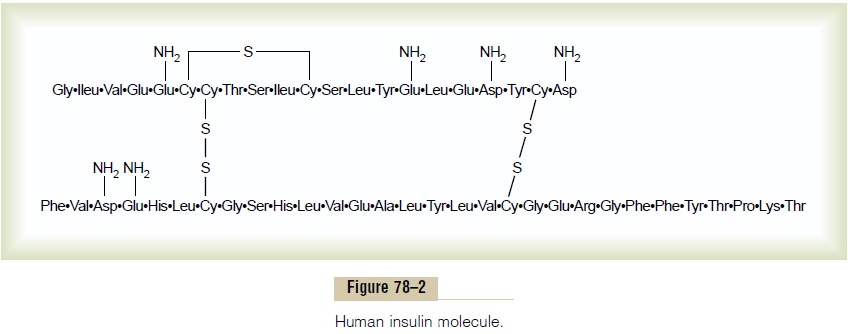
Insulin is a small protein; human insulin has a molec-ular weight of 5808. It is composed of two amino acid chains, shown in Figure 78–2, connected to each other by disulfide linkages. When the two amino acid chains are split apart, the functional activity of the insulin molecule is lost.
Insulin is synthesized in the beta cells by the usual cell machinery for protein synthesis, beginning with translation of the insulin RNA by ribosomes attached to the endoplasmic re-ticulum to form an insulin preprohormone. This initial preprohormone has a molecular weight of about 11,500, but it is then cleaved in the endoplasmic retic-ulum to form a proinsulin with a molecular weight of about 9000; most of this is further cleaved in the Golgi apparatus to form insulin and peptide fragments before being packaged in the secretory granules. However, about one sixth of the final secreted product is still in the form of proinsulin. The proinsulin has vir-tually no insulin activity.
When insulin is secreted into the blood, it circulates almost entirely in an unbound form; it has a plasma half-life that averages only about 6 minutes, so that it is mainly cleared from the circulation within 10 to 15 minutes. Except for that portion of the insulin that combines with receptors in the target cells, the remain-der is degraded by the enzyme insulinase mainly in the liver, to a lesser extent in the kidneys and muscles, and slightly in most other tissues. This rapid removal from the plasma is important, because, at times, it is as important to turn off rapidly as to turn on the control functions of insulin.
Activation of Target Cell Receptors by Insulin and the Resulting Cellular Effects
To initiate its effects on target cells, insulin first binds with and activates a membrane receptor protein that has a molecular weight of about 300,000 (Figure 78–3). It is the activated receptor, not the insulin, that causes the subsequent effects.
The insulin receptor is a combination of four sub-units held together by disulfide linkages: two alphasubunits that lie entirely outside the cell membraneand two beta subunits that penetrate through the mem-brane, protruding into the cell cytoplasm. The insulin binds with the alpha subunits on the outside of the cell, but because of the linkages with the beta subunits, the portions of the beta subunits protruding into the cell become autophosphorylated. Thus, the insulin receptor is an example of an enzyme-linked receptor. Autophosphorylation of the beta subunits of the receptor activates a local tyrosine
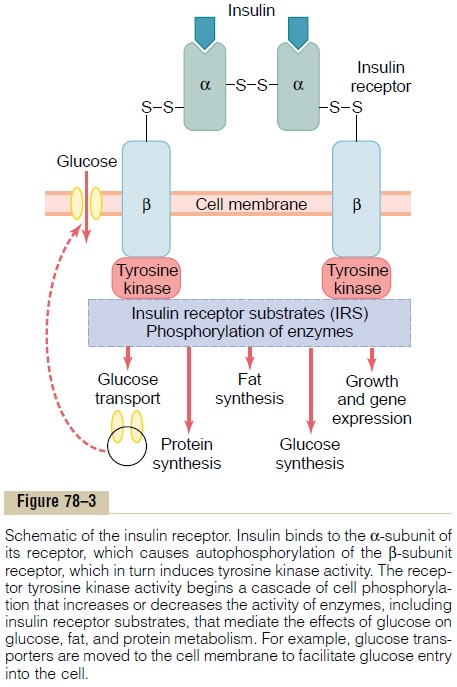
kinase, which in turn causes phosphorylation of multi-ple other intracellular enzymes including a group called insulin-receptor substrates (IRS). Different types of IRS (e.g. IRS-1, IRS-2, IRS-3) are expressed in different tissues. The net effect is to activate some of these enzymes while inactivating others. In this way, insulin directs the intracellular metabolic machinery to produce the desired effects on carbohydrate, fat, and protein metabolism. The end effects of insulin stimu-lation are the following:
1. Within seconds after insulin binds with its membrane receptors, the membranes of about 80 per cent of the body’s cells markedly increase their uptake of glucose. This is especially true of muscle cells and adipose cells but is not true ofmost neurons in the brain. The increased glucosetransported into the cells is immediately phosphorylated and becomes a substrate for all the usual carbohydrate metabolic functions. The increased glucose transport is believed to result from translocation of multiple intracellular vesicles to the cell membranes; these vesicles carry in their own membranes multiple molecules of glucose transport proteins, which bind with the cell membrane and facilitate glucose uptake into the cells. When insulin is no longer available, these vesicles separate from the cell membrane within about 3 to 5 minutes and move back to the cell interior to be used again and again as needed.
2. The cell membrane becomes more permeable to many of the amino acids, potassium ions, and phosphate ions, causing increased transport of these substances into the cell.
3. Slower effects occur during the next 10 to 15 minutes to change the activity levels of many more intracellular metabolic enzymes. These effects result mainly from the changed states of phosphorylation of the enzymes.
4. Much slower effects continue to occur for hours and even several days. They result from changed rates of translation of messenger RNAs at the ribosomes to form new proteins and still slower effects from changed rates of transcription of DNA in the cell nucleus. In this way, insulin remolds much of the cellular enzymatic machinery to achieve its metabolic goals.
Related Topics