Chapter: Medical Immunology: IgE-Mediated (Immediate) Hypersensitivity
IgE Antibodies
IgE Antibodies
Prausnitz and Küstner published the first demonstration that serum contains a factor capable of mediating specific allergic reactions in 1921. The injection of serum from a fish-al-lergic person (Küstner) into Dr. Prausnitz’s skin and subsequent exposure of Dr. Prausnitz to fish antigen injected in the same site resulted in an allergic wheal and flare response.
In 1967 Ishizaka and collaborators isolated a new class of immunoglobulin, desig-nated as IgE, from the serum of ragweed-allergic individuals. Several patients with IgE-producing plasmocytomas were subsequently discovered and provided a source of very large amounts of monoclonal IgE that greatly facilitated further studies of IgE structure and the production of anti-IgE antibodies.
1. Quantitative Assay of IgE Antibodies
The total IgE concentration, even in allergic individuals, is extremely low, not detectable by most routine assays used for the assay of IgG, IgA, and IgM. The concentration of spe-cific IgE antibody to any given allergen is a very small fraction of the total IgE.
Early attempts to measure IgE involved cumbersome and often unreliable bioassays. The availability of anti-IgE antibodies allowed the development of radioimmunoassays sufficiently sensitive to determine total serum IgE and IgE antibody levels accurately.
The paper disc radioimmunosorbent test (PRIST) was one of the first solid-phase ra-dioimmunoassays introduced in diagnostic medicine. This assay, dia-grammatically summarized in Figure 21.1, measures total serum IgE.
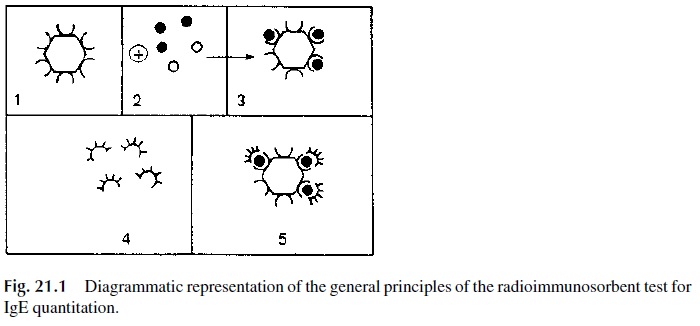
1. A serum sample is added to a small piece of adsorbent paper to which anti-IgE antibodies are covalently bound. The immobilized antibody captures IgE.
2. 125Iodine-labeled anti-IgE antibodies are subsequently added and will bind to the paper-bound-IgE. The radioactivity counted in the solid phase is directly related to the IgE level in the serum tested.
3. The results are expressed in nanograms/mL (1 ng = 10-6 mg) or in international units (1 I.U. = 2.5 ng/mL): 180 IU/mL is considered as the upper limit for nor-mal adults. Allergic individuals often have elevated levels of IgE. However, some asymptomatic individuals may also have elevated IgE levels. Therefore, a diagnosis of immediate hypersensitivity cannot be based solely on the determi-nation of abnormally elevated IgE levels.
Sensitive EIA and quantitative fluorescence assays were later developed that are equally able to measure total IgE levels without the need for use of radiolabeled com-pounds.
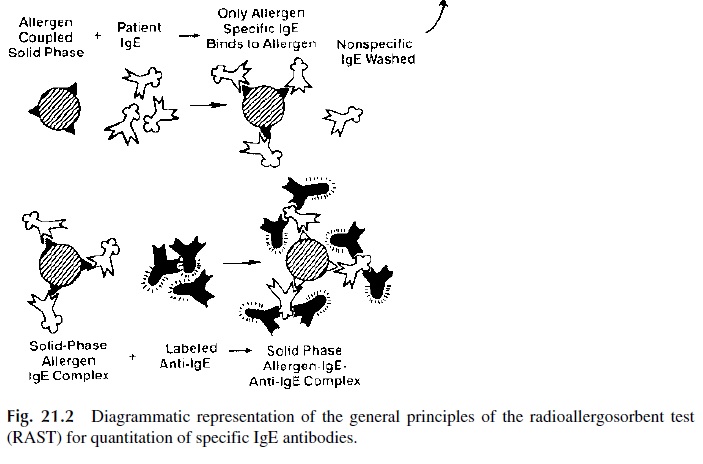
The radioallergosorbent test (RAST), diagrammatically summarized in Figure 21.2, is a solid phase radioimmunoassay that determines antigen-specific IgE, which from the di-agnostic point of view is considerably more relevant than the measurement of total serum IgE levels.
1. A given allergen (ragweed antigen, penicillin, β-lactoglobulin, etc.) is covalently bound to polydextran beads
2. Patient’s serum is added to beads coated with a single antigen; the antigen-spe-cific IgE, if present, will bind to the immobilized antigen.
3. After washing off unbound immunoglobulins, radiolabeled anti-IgE is added. The amount of bead-bound radioactivity counted after washing off unbound la-beled antibody is directly related to the concentration of antigen-specific IgE present in the serum.
2. Skin Tests
Although the RAST assays are highly specific and accurate, they are expensive and lack sensitivity, and the range of antigens for which there are available tests is limited. In addi-tion, some authors have cast doubts about the biological relevance of the RAST assay re-sults. The alternative method for diagnosis of specific allergies is provocation skin tests, which allow testing to a wider array of antigens. Although positive skin tests depend on the existence of IgE antibodies, they do not allow a direct quantitative assay of such antibod-ies; rather, they provide information about their ability to mediate the hypersensitivity reaction. This explains the opinion of many specialists that the results of skin tests correlate better with clinical data than the results of the RAST assays.
The skin tests for immediate hypersensitivity are performed by injecting small amounts of purified allergens percutaneously or intradermally in known patterns. The pa-tients are then observed for about 30 minutes to one hour. Classical IgE-mediated hyper-sensitivity reactions present as a wheal and flare at the site of the allergen exposure, which develops in a matter of minutes.
In highly sensitized individuals, there is always a risk of anaphylaxis, even after minimal challenge. Because of this risk, trained professionals should always perform these tests in a property equipped clinical facility.
Related Topics