Chapter: Biochemistry: Storage Mechanisms and Control in Carbohydrate Metabolism
How is glycogen metabolism controlled?
How is glycogen metabolism
controlled?
How does an organism ensure that glycogen synthesis and glycogen
breakdown do not operate simultaneously? If this were to occur, the main result
would be the hydrolysis of UTP, which would waste chemical energy stored in the
phosphoric anhydride bonds. A major controlling factor lies in the behavior of
glycogen phosphorylase. This enzyme is subject not only to allosteric control but
also to another control feature: covalent modification. In that example,
phosphorylation and dephosphorylation of an enzyme determined whether it was
active, and a similar effect takes place here.
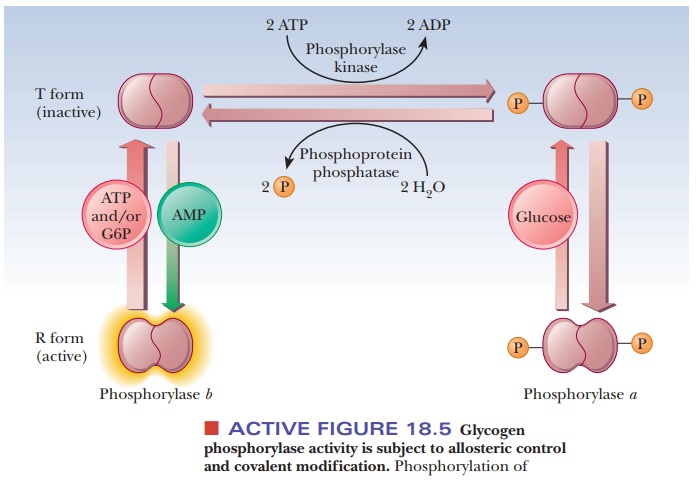
Figure 18.5 summarizes some of the salient control features that affect glyco-gen phosphorylase activity. The enzyme is a dimer that exists in two forms, the inactive T (taut) form and the active R (relaxed) form. In the T form (and only in the T form), it can be modified by phosphorylation of a specific serine resi-due on each of the two subunits. The esterification of the serines to phosphoric acid is catalyzed by the enzyme phosphorylase kinase; the dephosphorylation is catalyzed by phosphoprotein phosphatase. The phosphorylated form of glycogen phosphorylase is called phosphorylasea, and the dephosphorylated form is called phosphorylaseb. The switch from phosphorylase b to phosphorylase a is the major form of control over the activity of phosphorylase. The response time of the changes is on the order of seconds to minutes. Phosphorylase is also controlled more quickly in times of urgency by allosteric effectors, with a response time of milliseconds.
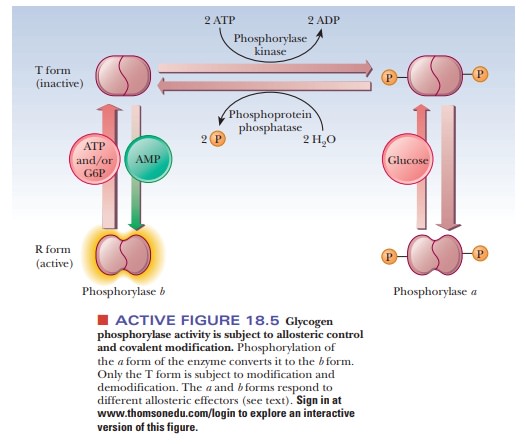
In liver, glucose is an allosteric inhibitor of
phosphorylase a. It binds to the
substrate site and favors the transition to the T state. It also exposes the
phosphorylated serines so that the phosphatase can hydrolyze them. This shifts
the equilibrium to phosphorylase b.
In muscle, the primary allosteric effectors are ATP, AMP, and
glucose-6-phosphate (G6P). When the muscles use ATP to contract, AMP levels
rise. This increase in AMP stimulates formation of the R state of phosphorylase
b, which is active. When ATP is
plentiful or glucose-6-phosphate builds up, these molecules act as allosteric
inhibitors shifting the equilibrium back to the T form. These differences
ensure that glycogen will be degraded when there is a need for energy, as is
the case with high [AMP], low [G6P], and low [ATP]. When the reverse is true
(low [AMP], high [G6P], and high [ATP]), the need for energy, and consequently
for glycogen breakdown, is less. “Shutting down” glycogen phosphorylase
activity is the appropriate response. The combination of covalent modification
and allosteric control of the process allows for a degree of fine-tuning that
would not be possible with either mechanism alone. Hormonal control also enters
into the picture. When epinephrine is released from the adrenal gland in
response to stress, it triggers a series of events, that suppress the activity
of glycogen synthase and stimulate that of glycogen phosphorylase.
The activity of glycogen synthase is subject to
the same type of covalent modification as glycogen phosphorylase. The
difference is that the response is opposite. The inactive form of glycogen
synthase is the phosphorylated form. The active form is unphosphorylated. The
hormonal signals (glucagon or epi-nephrine) stimulate the phosphorylation of
glycogen synthase via an enzyme called cAMP-dependent protein kinase. After the
glycogen syn-thase is phosphorylated, it becomes inactive at the same time the
hormonal sig-nal is activating phosphorylase. Glycogen synthase can also be
phosphorylated by several other enzymes, including phosphorylase kinase and
several enzymes called glycogen synthase kinases. Glycogen synthase is
dephosphorylated by the same phosphoprotein phosphatase that removes the
phosphate from phos-phorylase. The phosphorylation of glycogen synthase is also
more complicated in that there are multiple phosphorylation sites. As many as
nine different amino acid residues have been found to be phosphorylated. As the
progressive level of phosphorylation increases, the activity of the enzyme
decreases.
Glycogen synthase is also under allosteric
control. It is inhibited by ATP. This inhibition can be overcome by
glucose-6-phosphate, which is an activa-tor. However, the two forms of glycogen
synthase respond very differently to glucose-6-phosphate. The phosphorylated
(inactive) form is called glycogensynthase
D (for “glucose-6-phosphate dependent”) because it is active onlyunder very
high concentrations of glucose-6-phosphate. In fact, the level necessary to
give significant activity would be beyond the physiological range. The
nonphosphorylated form is called glycogen
synthase I (for “glucose-6-phosphate independent”) because it is active
even with low concentrations of glucose-6-phosphate. Thus, even though purified
enzymes can be shown to respond to allosteric effectors, the true control over
the activity of glycogen syn-thase is by its phosphorylation state, which, in
turn, is controlled by hormonal states.
The fact that two target enzymes, glycogen
phosphorylase and glycogen syn-thase, are modified in the same way by the same
enzymes links the opposing processes of synthesis and breakdown of glycogen
even more intimately.
Finally, the modifying enzymes are themselves subject to covalent modifica-tion and allosteric control. This feature complicates the process considerably but adds the possibility of an amplified response to small changes in conditions. A small change in the concentration of an allosteric effector of a modifying enzyme can cause a large change in the concentration of an active, modified target enzyme; this amplification response is due to the fact that the substrate for the modifying enzyme is itself an enzyme.
At this point, the situation has become very complex indeed, but it is a good
example of how opposing pro-cesses of breakdown and synthesis can be controlled
to the advantage of an organism.
Related Topics