Chapter: Modern Pharmacology with Clinical Applications: Gene Therapy
Gene Therapy
Gene
Therapy
Most drugs used today are
designed to treat symp-toms rather than cure the underlying disease. Notable
exceptions include cytotoxic chemotherapeutic agents and agents that restore or
modulate hormone function. However, increased understanding of the molecular
and genetic etiology of diseases may permit permanent modification of organ
function by drug-oriented meth-ods. The first disease-associated gene, -globin,
was cloned over 25 years ago. It is now theoretically possible to isolate,
sequence, and analyze genes causally associ-ated with many heritable and
acquired human diseases, including cystic fibrosis, Duchenne’s muscular
dystro-phy, and Gaucher’s disease. Moreover, with the com-plete sequencing of
the human genome, many of the es-timated 100,000 human genes may become
candidates for genetic manipulations. Thus, it is now possible to propose
molecular pharmacological and genetic ap-proaches to therapy . Many of these
approaches fall un-der the general rubric of gene therapy.
Germ cell gene therapy will
require considerable dis-cussion about ethical issues and extensive information
before it can be applied to humans, but somatic cell gene therapy in humans is
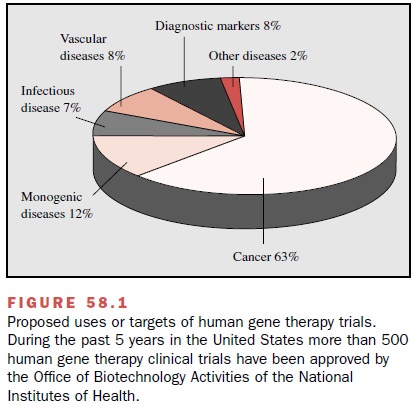
now being extensively explored. During the past 5 years in the United States
alone, more than 500 human gene therapy clinical trials aimed at treating
conditions ranging from inherited disorders such as cystic fibrosis to cancer
and AIDS, have been ap-proved by the Office of Biotechnology Activities (OBA,
formerly the Recombinant DNA Advisory Committee) of the National Institutes of
Health. Nearly 3500 patients have been enrolled in these studies (Fig. 58.1).
With few exceptions, gene
therapy was considered safe if not particularly effective until the death of an
18-year-old man in 1999, the first fatal outcome for a pa- tient in a phase I
gene therapy protocol. This death has stimulated a substantial review of the
oversight mecha-nisms in human gene transfer research. One of the first
successes of gene therapy was reported in 2000, when three infants with a fatal
form of severe combined im-munodeficiency syndrome (SCID) received ex vivo gene
therapy with a recombinant mouse leukemia viral vector encoding the γC receptor gene. After 10
months, γC transgene expression in T- and NK cells was de-tected and T-, B-,
and NK-cell counts and function were comparable to those of age-matched
controls.
Although numerous obstacles
must be overcome before gene therapy will be routinely employed, a rig-orous
approach to investigating the safety and efficacy of gene transfer will ensure
that clinical strategies em-ploying genetic manipulation are rationally
incorpo-rated into the therapeutic armamentarium.
Related Topics