Chapter: Modern Pharmacology with Clinical Applications: Gene Therapy
Gene Therapy: Delivery Systems
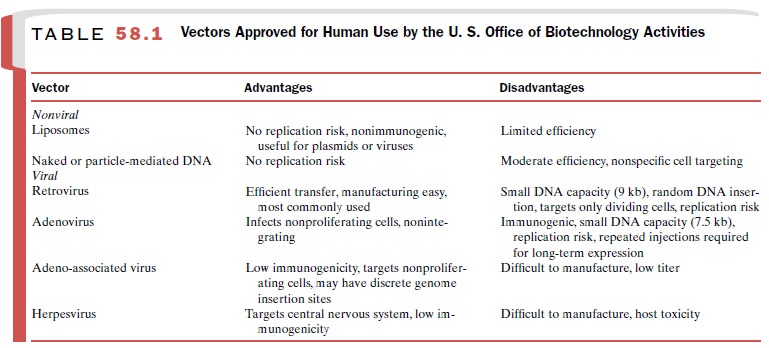
DELIVERY SYSTEMS
In many cell types it is
feasible to deliver nucleic acids and genes by a variety of methods when the
cells are grown in tissue culture (Table 58.1). Nonetheless, some cells, such
as pneumocytes and neurons, are not readily isolated from humans and do not
grow well in vitro. Furthermore, for many diseases it is essential to alter the
phenotype of a significant proportion of the total cell population, making ex
vivo gene therapy of limited use.
There is general agreement
that no ideal delivery system is available for in vivo gene therapy. Direct or
in-tratumoral injection of plasmid DNA or antisense oligomers without a viral
vector has been attempted. Expression of genes using traditional nonviral
vectors has been low compared to viral strategies. Nonetheless, recent
breakthroughs in nonviral delivery systems, in-cluding the gene gun,
electroporation and naked DNA, suggest that nonviral gene therapy can achieve
local ex-pression of therapeutic genes at levels equivalent to those of viral
vectors.
Although the mechanism
remains undetermined, the injection of naked DNA into skeletal muscle has
demon-strated relatively high transfection efficiency. In this set-ting, DNA is
precipitated onto the surface of microscopic metal beads (e.g., gold) and the
microprojectiles are ac-celerated and penetrate intact tissue to several cell
layers.
In preclinical trials,
efficiency remains low, but expression has been noted to last for several
weeks, and there has been no significant inflammatory response.
Some investigators have used
electrical current (electroporation)
to improve DNA (or drug) entry into tumor cells with some preliminary success.
Liposomes are attractive vehicles for gene delivery, since they can carry
plasmid, antisense, or viral DNA. Compared with viral approaches, however,
liposomes remain relatively inefficient at facilitating gene transfer, although
their safety profile remains more desirable. Some of the at-tributes and
limitations of the nonviral methods are listed in Table 58.1.
Because viruses can
efficiently integrate into the genome, many clinical trials are exploring the
use of replication-defective recombinant
viral vectors and de-livery systems. Retroviruses contain their genetic
infor-mation as a double-strand DNA genome that is tran-scribed, and the
single-strand proviral DNA product is stably integrated into the host genome.
Recombinant DNA technology has been used to remove deleterious viral genes
involved in replication, and the resulting vector is replication defective,
nonpathogenic, and un-able to produce infectious particles. Ideally, with a
retro-viral vector, only a single administration should be re-quired because
the gene should be permanently retained and expressed. No clinical evidence of
mutage-nesis has emerged from the clinical trials performed to date, but the
number of patients treated and the time of exposure has been limited.
Adenoviral vectors have also been used in human trials. These vectors enter cells by
either an adenovirus fiber–specific receptor or a surface integrin receptor.
They efficiently transfer genes in nonreplicating and replicating cells.
Nonetheless, immunological responses to viruses have been noted with adenoviral
vectors. Replication-selective adenovirus
vectors have been in-troduced to optimize infection of target cells and
mini-mize infection of normal cells. Over 200 cancer patients have been treated
to date in more than 10 clinical trials with little evidence of toxicity
reported. Replication, however, has generally been transient ( 10 days), with
limited efficacy observed when the gene therapy was administered as a single
agent. More encouraging anti-tumor effects have been observed when the gene
ther-apy was combined with cytotoxic chemotherapy. Further modifications are
likely to be required before there can be general application of adenoviral
vectors for cancer therapy.
Related Topics