Chapter: Health Management in Aquaculture: Immunological and molecular biology techniques in disease diagnosis
Enzyme-Linked Immunosorbent Assay (ELISA) - Aquaculture Immunological techniques in disease diagnosis
Enzyme-Linked Immunosorbent Assay (ELISA) : The covalent attachment of enzymes to antibody molecules creates an immunological tool possessing both high specificity and sensitivity. The technique makes use of antibodies to which enzymes have been covalently bound suchthat the enzyme’s catalytic properties and the antibody’s specificity are unal-tered. Typical linked enzymes include peroxidase, alkaline phosphatase and b-galactosidase, all of which catalyze reactions whose products are colored andcan be measured in very low amounts.
The ELISA is one of the most powerful of all immunochemical techniques. It employs a wide range of methods to detect and quantitate antigens or antibod-ies and to study the structure of antigens. There are many variations on the ways the immunoassays can be performed. Immunoassays are classified on the basis of methodology and within each group, the principle and the order of the steps are similar. For example, by changing certain key conditions, an as-say can be altered to determine either antigen or antibody level. Although the steps are similar, the assays yield different results.
The three classes of ELISA tests are:
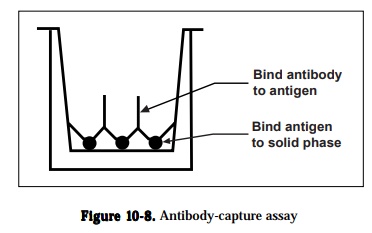
1) antibody capture assays,
2) antigen capture assays and
3) two-antibody sandwich assays
Antibody capture assay can be used to detect and quantitate antigens or anti-bodies and compare the epitopes recognized by different antibodies. The gen-eral protocol; an unlabeled antigen is immobilized on a solid phase and the antibody is allowed to bind to the immobilized antigen (Figure 10-8). The antibody can be labeled directly or can be detected by using a labeled second-ary reagent like goat anti-rabbit or anti-mouse IgG antibody containing conju-gated enzyme that will specifically recognize the antibody. Following the addi-tion of enzyme substrate, a color is formed, and the amount of antibody rela-tive to the specific antigen is quantitated from the intensity of the color reac-tion measured by an ELISA plate reader, a modified spectrophotometer The color formed is proportional to the amount of antibody that is bound. The three factors that will affect the sensitivity of a labeled antibody assay are (1) the amount of antigen that is bound to the solid phase, (2) the avidity of the antibody for the antigen and (3) the type and number of labeled moieties used to label the antibody. Variations in methodology under antibody capture assay are (1) detecting and quantitating antibodies using antigen excess assays, (2) comparing antibody binding sites using an antibody competition assay, (3) de-tecting and quantitating antigens using antibody excess assays and (4) detect-ing and quantitating antigens using antigen competition assays.
Antigen capture assays are used primarily to detect and quantitate antigens(Figure 10-9). The amount of antigen in the test solution is determined using a competition between labeled and unlabeled antigen. Unlabeled antibody is bound to the solid phase either directly or through an intermediate protein, such as an anti-immunoglobulin antibody. The antigen is purified and labeled. A sample of the labeled antigen is mixed with the test solution containing an unknown amount of antigen and the mixture is added to the bound antibody.
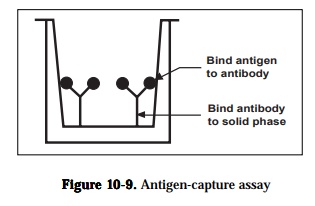
known solution contains a high concentration of antigen, it will compete effec-tively with the labeled antigen and little or none of the labeled antigen will bind to the antibody. Following a wash, the enzyme activity of the bound material in each microtiter well is determined by adding the substrate of the enzyme. Color development in individual wells of the plate is assessed by eye or more commonly, quantified with a commercially available ELISA plate reader-a modified spectrophotometer The color formed is proportional to the amount of antigen originally present. It is necessary to determine cut-off val-ues to discriminate between true positive reactions and background reactivity by including appropriate positive and negative controls with each series of as-says. The sensitivity of the labeled antigen assay will depend on three factors:(1) the number of antibodies that are bound to the solid phase, (2) the avidity of the antibody for the antigen and (3) the specific activity of the labeled anti-gen.
Two-antibody sandwich assays are used primarily to determine the antigenconcentration in unknown samples. The assay requires two antibodies that bind to non-overlapping epitopes on the antigen (Figure 10-10). Either two monoclonal antibodies that recognize discrete sites or one batch of affinity-purified polyclonal antibodies can be used. To use the assay, one antibody is
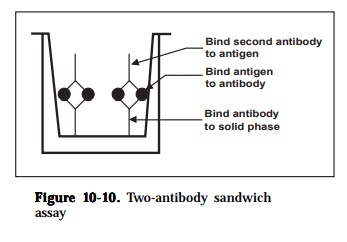
purified and bound to a solid phase and the antigen in a test solution is allowed to bind. Unbound proteins are removed by washing and the labeled second antibody is allowed to bind to the antigen. The conjugate comprises antibody chemically linked to the enzyme horseradish peroxidase. After a further incu-bation, excess conjugate is removed by washing the wells and the bound per-oxidase is determined by adding chromogen in substrate buffer. Color develop-ment (blue) is proportional to the original bacterial content in the sample and can easily be read by the naked eye. The assay can also be amplified at this stage thus increasing sensitivity by adding stop solution. The color obtained changes from blue to yellow and results are read using an ELISA plate reader with a 450 nm filter. The inclusion of appropriate positive and negative con-trols are essential. In addition, during the optimization of the test, check for endogenous peroxidase activity in the tissue samples being used. If activity is found, then this may be overcome by altering the method of extraction (e.g. heating step). It may be necessary to change to a different enzyme system (e.g. alkaline phosphatase) if activity is very high (e.g. spleen tissue). The major advantages of this method are that the antigen does not need to be purified prior to use and that the assays are very specific. The major disadvantage is that not all antibodies can be used. The sensitivity of the assay is dependent on four factors: (1) the number of molecules of the first antibody that are bound to the solid phase, (2) the avidity of the first antibody for the antigen,(3) the avidity of the second antibody for the antigen and (4) the specific activ-ity of the labeled second antibody.
Two types of detection systems are commonly used for ELISA: iodinated re-agents and enzyme-labeled reagents. Assays that use iodinated reagents are easier to quantitate while enzyme assays will often yield a quicker result. Ei-ther iodinated or enzyme-labeled reagents can be used for direct or indirect methods. When using direct detection methods, the antibody or antigen is purified and labeled while for indirect detection, a labeled secondary reagent that will bind specifically to an antibody is used. A third variation that uses properties of both direct and indirect detection is the biotin-streptavidin sys-tem. Here, the antigen or antibody is purified and labeled with biotin. The biotinylated reagent is detected by binding with streptavidin that has been la-beled with iodine or an enzyme.
ELISA test has been used to detect Aeromonas salmonicida in fish tissue (Adams, 1990), clinical cases of enteric red mouth and furunculosis in fish farms (Austin et al., 1986), Vibrio parahaemolyticus (Adams, 1991) and V.harveyi (Song et al., 1992) in penaeid shrimp. ELISA has also been developedfor rapid detection of viral haemorrhagic septicaemia virus (Olesen and Jorgensen, 1991) and striped jack nervous necrosis virus (Arimoto et al., 1992) in fish.
Related Topics