Chapter: Medical Physiology: Insulin, Glucagon, and Diabetes Mellitus
Effect of Insulin on Fat Metabolism
Effect of Insulin on Fat Metabolism
Although not quite as visible as the acute effects of insulin on carbohydrate metabolism, insulin’s effects on fat metabolism are, in the long run, equally important. Especially dramatic is the long-term effect of insulin lack in causing extreme atherosclerosis, oftenleading to heart attacks, cerebral strokes, and other vascular accidents. But first, let us discuss the acute effects of insulin on fat metabolism.
Insulin Promotes Fat Synthesis and Storage
Insulin has several effects that lead to fat storage in adipose tissue. First, insulin increases the utilization of glucose by most of the body’s tissues, which automatically decreases the utilization of fat, thus functioning as a fat sparer. However, insulin also promotes fatty acid synthesis. This is especially true when more carbohydrates are ingested than can be used for immediate energy, thus providing the sub-strate for fat synthesis. Almost all this synthesis occurs in the liver cells, and the fatty acids are then trans-ported from the liver by way of the blood lipoproteins to the adipose cells to be stored. The different factors that lead to increased fatty acid synthesis in the liver include the following:
1. Insulin increases the transport of glucose into the liver cells. After the liver glucogen concentrationreaches 5 to 6 per cent, this in itself inhibits further glycogen synthesis. Then all the additional glucose entering the liver cells becomes available to form fat. The glucose is first split to pyruvate in the glycolytic pathway, and the pyruvate subsequently is converted to acetyl coenzyme A (acetyl-CoA), the substrate from which fatty acids are synthesized.
2. An excess of citrate and isocitrate ions is formed by the citric acid cycle when excess amounts of glucose are being used for energy. These ions thenhave a direct effect in activating acetyl-CoAcarboxylase, the enzyme required to carboxylateacetyl-CoA to form malonyl-CoA, the first stage of fatty acid synthesis.
3. Most of the fatty acids are then synthesized within the liver itself and used to form triglycerides, theusual form of storage fat. They are released from the liver cells to the blood in the lipoproteins. Insulin activates lipoprotein lipase in the capillary walls of the adipose tissue, which splits the triglycerides again into fatty acids, a requirement for them to be absorbed into the adipose cells, where they are again converted to triglycerides and stored.
Role of Insulin in Storage of Fat in the Adipose Cells. Insulinhas two other essential effects that are required for fat storage in adipose cells:
1. Insulin inhibits the action of hormone-sensitive lipase. This is the enzyme that causes hydrolysis ofthe triglycerides already stored in the fat cells. Therefore, the release of fatty acids from the adipose tissue into the circulating blood is inhibited.
2. Insulin promotes glucose transport through the cell membrane into the fat cells in exactly the sameways that it promotes glucose transport into muscle cells. Some of this glucose is then used to synthesize minute amounts of fatty acids, but more important, it also forms large quantities of a-glycerol phosphate. This substance supplies the glycerol that combines with fatty acids to formthe triglycerides that are the storage form of fat in adipose cells. Therefore, when insulin is not available, even storage of the large amounts of fatty acids transported from the liver in the lipoproteins is almost blocked.
Insulin Deficiency Increases Use of Fat for Energy
All aspects of fat breakdown and use for providing energy are greatly enhanced in the absence of insulin. This occurs even normally between meals when secre-tion of insulin is minimal, but it becomes extreme in diabetes mellitus when secretion of insulin is almost zero. The resulting effects are as follows.
Insulin Deficiency Causes Lipolysis of Storage Fat and Release of Free Fatty Acids. In the absence of insulin, all theeffects of insulin noted earlier that cause storage of fat are reversed. The most important effect is that the enzyme hormone-sensitive lipase in the fat cells becomes strongly activated. This causes hydrolysis of the stored triglycerides, releasing large quantities of fatty acids and glycerol into the circulating blood. Consequently, the plasma concentration of free fatty acids begins to rise within minutes. This free fatty acid then becomes the main energy substrate used by essentially all tissues of the body besides the brain.
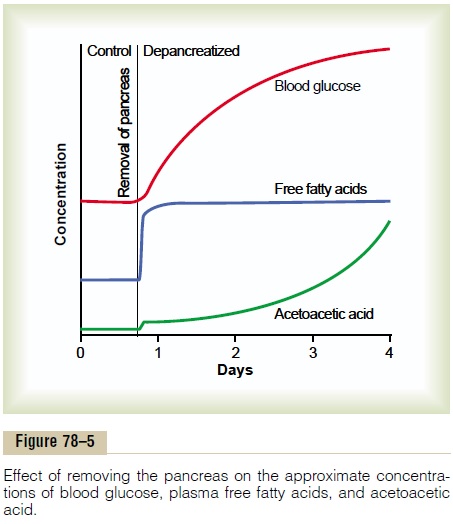
Figure 78–5 shows the effect of insulin lack on the plasma concentrations of free fatty acids, glucose, and acetoacetic acid. Note that almost immediately after removal of the pancreas, the free fatty acid concentra-tion in the plasma begins to rise, more rapidly even than the concentration of glucose.
Insulin Deficiency Increases Plasma Cholesterol and Phospho-lipid Concentrations. The excess of fatty acids in theplasma associated with insulin deficiency also pro-motes liver conversion of some of the fatty acids into phospholipids and cholesterol, two of the major prod-ucts of fat metabolism. These two substances, along with excess triglycerides formed at the same time in the liver, are then discharged into the blood in the lipoproteins. Occasionally the plasma lipoproteins increase as much as threefold in the absence of insulin, giving a total concentration of plasma lipids of several per cent rather than the normal 0.6 per cent. This high lipid concentration—especially the high concentration of cholesterol—promotes the development of athero-sclerosis in people with serious diabetes.
Excess Usage of Fats During Insulin Lack Causes Ketosis and Acidosis. Insulin lack also causes excessive amounts ofacetoacetic acid to be formed in the liver cells. Thisresults from the following effect: In the absence of insulin but in the presence of excess fatty acids in the liver cells, the carnitine transport mechanism for trans-porting fatty acids into the mitochondria becomes increasingly activated. In the mitochondria, beta oxi-dation of the fatty acids then proceeds very rapidly, releasing extreme amounts of acetyl-CoA.A large part of this excess acetyl-CoA is then condensed to form acetoacetic acid, which in turn is released into the cir-culating blood. Most of this passes to the peripheral cells, where it is again converted into acetyl-CoA and used for energy in the usual manner.
At the same time, the absence of insulin also depresses the utilization of acetoacetic acid in the peripheral tissues. Thus, so much acetoacetic acid is released from the liver that it cannot all be metabo-lized by the tissues. Therefore, as shown in Figure 78–5, its concentration rises during the days after cessation of insulin secretion, sometimes reaching concentra-tions of 10 mEq/L or more, which is a severe state of body fluid acidosis.
Some of the acetoacetic acid is also converted into b-hydroxybutyric acid and acetone. These two substances, along with the ace-toacetic acid, are called ketone bodies, and their pres-ence in large quantities in the body fluids is called ketosis. We see later that in severe diabetes the ace-toacetic acid and the b-hydroxybutyric acid can cause severe acidosis and coma, which often leads to death.
Related Topics