Chapter: Organic Chemistry: Amines and nitriles
Chemistry of nitriles
CHEMISTRY OF NITRILES
Key Notes
Preparation
Primary
alkyl halides can be treated with the cyanide ion and converted to nitriles of
general formula RCH2CN. Primary amides can be dehydrated with
thionyl chloride or phosphorus pentoxide to give a nitrile.
Properties
The
nitrile group is linear in shape with both the carbon and nitrogen being sp hybridized. The triple bond is made
up of oneσbond and
twoπbonds.The
nitrile group is polarized such that the nitrogen is a nucleophilic center and
the carbon is an electrophilic center. Nucleophiles react with nitriles at the
electrophilic carbon center to form an imine intermediate which reacts further
depending on the reaction conditions.
Reactions
Nitriles
can be hydrolyzed to carboxylic acids on treatment with aqueous acid or base.
The reaction involves the formation of an intermediate pri-mary amide. Nitriles
can be converted to primary amines by reduction with lithium aluminum hydride.
The reaction involves addition of two hydride ions. With a milder reducing
agent such as DIBAH, only one hydride ion is added and an aldehyde is obtained.
Nitriles react with Grignard reagents to produce ketones. The Grignard reagent
provides the equivalent of a car-banion which reacts with the electrophilic
carbon center of the nitrile group.
Spectroscopic analysis
The
presence of a nitrile group is indicated by a CN stretching absorption in the
IR spectrum and a quaternary carbon signal in the 13C nmr spectrum.
Preparation
Nitriles are commonly prepared by the SN2
reaction of a cyanide ion with a primary alkyl halide. However, this limits the
nitriles which can be synthesized to those having the following general formula
RCH2CN. A more general synthesis of nitriles involves the
dehydration of primary amides with reagents such as thionyl chloride or
phosphorus pentoxide (Fig. 1).
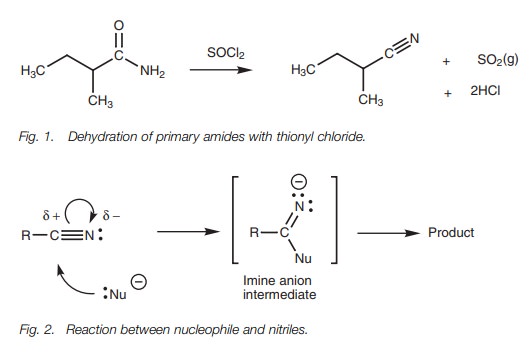
Properties
The nitrile group (CN) is linear in shape with both the carbon and the nitrogen atoms being sp hybridized. The triple bond linking the two atoms consists of one σ bond and two π bonds. Nitriles are strongly polarized. The nitrogen is a nucleophilic center and the carbon is an electrophilic center. Nucleophiles react with nitriles at the electrophilic carbon (Fig. 2). Typically, the nucleophile will forma bond to the electrophilic carbon resulting in simultaneous breaking of one of the bonds. The π electrons end up on the nitrogen to form ansp2 hybridized imine anion which then reacts further to give different products depending on the reaction conditions used.
Reactions
Nitriles (RCN) are hydrolyzed to carboxylic
acids (RCO2H) in acidic or basic aqueous solutions. The mechanism of
the acid-catalyzed hydrolysis (Fig. 3)
involves initial protonation of the nitrile’s nitrogen atom. This activates the
nitrile group towards nucleophilic attack by water at the electrophilic carbon.
One of the nitrile π bonds breaks simultaneously and both the π electrons move onto the nitrogen resulting in a hydroxy imine.
This rapidly isomerizes to a primary amide which is hydrolyzed under the
reaction conditions to give the
carboxylic acid and ammonia.
Nitriles (RCN) can be reduced to primary amines
(RCH2NH2) with lithium alu-minum hydride which provides
the equivalent of a nucleophilic hydride ion. The reaction can be explained by
the nucleophilic attack of two hydride ions (Fig. 4).
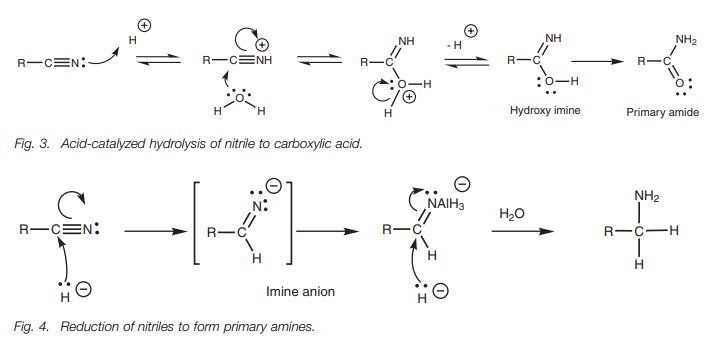
With a milder reducing agent such as DIBAH
(diisobutylaluminum hydride), the reaction stops after the addition of one
hydride ion, and an aldehyde is obtained instead (RCHO).
Grignard reaction
Nitriles can be treated with Grignard reagents
or organolithium reagents to give
ketones (Fig. 5).
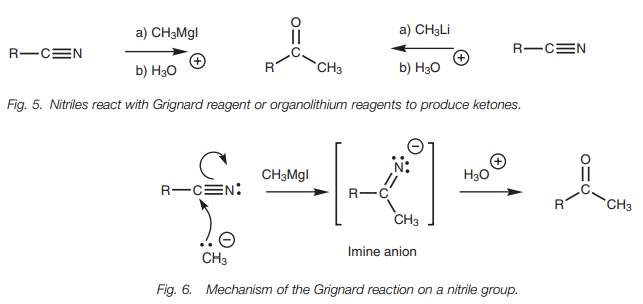
Grignard reagents provide the equivalent of a nucleophilic carbanion which can attack the electrophilic carbon of a nitrile group (Fig. 6). One of the π bonds is broken simultaneously resulting in an intermediate imine anion which is converted to a ketone when treated with aqueous acid.
Spectroscopic analysis
The IR spectrum of an aliphatic nitrile shows
an absorption due to stretching of the nitrile group in the region 2260–2240 cm−1. The corresponding absorption for an aromatic
nitrile is in the region 2240–2190 cm−1. These regions are normally devoid of
absorptions and so any absorption here can usually be identified.
The carbon of the nitrile group gives a
quaternary signal in the 13C nmr spectrum which is typically in the
region 114–124 ppm.
If a single nitrile group is present, the
molecular ion in the mass spectrum must be an odd number due to the presence of
nitrogen.
Related Topics