Chapter: Clinical Cases in Anesthesia : Thoracic Trauma
What are the clinical implications of blunt cardiac trauma?
What are the clinical implications of blunt cardiac trauma?
By definition, blunt cardiac trauma encompasses
a wide variety of pathologic conditions, including varying degrees of
myocardial damage; coronary artery injury; cardiac free wall, interatrial or
interventricular septal or valvular ruptures; and, with impalement by a
fractured sternum or rib, a penetrating injury from blunt trauma. The term
blunt cardiac injury (BCI), formerly called myocardial contusion, refers to
myocardial damage involving myofibrillar disinte-gration, edema, bleeding, or
necrosis that, depending on its severity, clinically presents as minor ECG or
cardiac enzyme abnormalities, complex dysrhythmias, or cardiac pump fail-ure.
Some of these injuries may be caused by myocardial damage from direct
mechanical injury or indirectly as a result of coronary occlusion or
exacerbation of pre-existing coronary artery disease by the stress of trauma.
Cardiac lesions, seen as ECG abnormalities and troponin release, may also be
the result of increased catecholamine activity following severe central nervous
system injury and hemor-rhagic shock with little or no direct trauma to the
heart.
In major trauma, multiple injuries frequently
coexist with BCI. The perioperative physician must be able to:
· anticipate the possibility of cardiac-induced
hemodynamic and rhythm abnormalities that may occur during the clinical course
of a patient who may initially be stable;
· determine the contribution of cardiac trauma to
the existing overall circulatory abnormality caused by hemorrhage, hypothermia,
acid–base and electrolyte abnormalities, or various other causes; and implement the most appropriate management based on these findings.
The
incidence of BCI in blunt thoracic trauma is approximately 20%, although it may
be as high as 76% in severe injuries. Nevertheless, major cardiac complications
requiring treatment are considerably less likely to occur; however, when they
do occur they may be fatal.
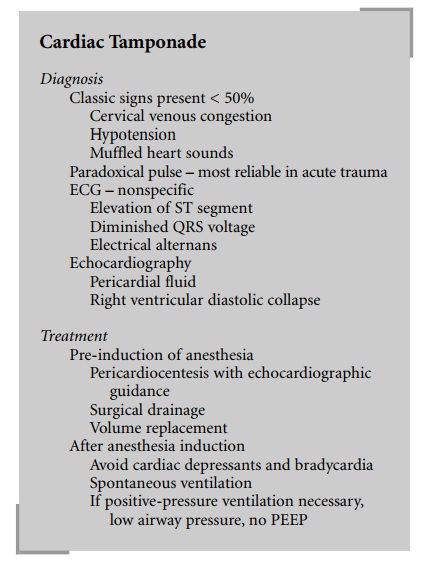
Diagnosis
BCI may be associated with direct precordial impact,
crush injury of the chest causing compression of the heart between the sternum
and the spine, deceleration injuries, and blast injuries. Suggestive signs and
symptoms include chest pain, angina responding to nitroglycerine, dyspnea,
chest wall ecchymosis, and fractures of ribs and/or sternum. There is no gold
standard for diagnosing blunt cardiac trauma. However, ECG, blood concentration
of troponin I, and echocardiogra-phy are important. In addition, coronary
angiography or ventriculography may very rarely be indicated to diagnose
coronary lesions, cardiac rupture, or valve injuries.
A 12-lead ECG is the best screening test.
Nonspecific changes such as tachycardia, bradycardia, or occasional atrial or
ventricular premature contractions occur in 50–70% of patients, and usually do
not require treatment. More serious alterations such as ST or T wave changes,
conduction delays, and complex atrial and ventricular dysrhythmias occur less
frequently (4–30%) but often need to be treated. Although sensitive, the ECG is
not specific and a normal trace cannot rule out the diagnosis. Because of its
anterior position, the right ventricle is injured more frequently than the
left. However, standard leads, which are primarily useful to detect left-sided
abnormalities, will miss some right heart events. Overall the sensitivity,
speci-ficity, and negative predictive value of ECG for predicting cardiac
complications are 100%, 47%, and 90%, respec-tively. Although delayed (>24
hours) appearance of serious ECG abnormalities may very rarely occur, it has
been demonstrated that a normal ECG on admission virtually eliminates the risk
of complications of blunt cardiac trauma and the need for further evaluation,
as long as the patient is hemodynamically stable, has no history of cardiac
disease, is less than 55 years old, and does not have multiple injuries or
significant chest wall trauma. If these compromising condi-tions exist,
however, cardiac monitoring for at least 24 hours in a telemetry ward or
intensive care unit is indicated. Of course, an abnormal ECG should be followed
by contin-uous cardiac monitoring. Additionally, obtaining serum tro-ponin I
concentration 6 and 12 hours after the injury in hemodynamically stable
patients, and echocardiography in hemodynamically unstable patients, should be
considered.
Serum creatine kinase (CK) and creatine kinase
MB (CK-MB) determinations, which were frequently used to diagnose myocardial
contusion, are no longer performed because of their low specificity. Skeletal
muscle, colon, lung, liver, and pancreatic tissue contain both CK and CK-MB.
Thus in multiple trauma, a positive value may not indicate cardiac injury.
Troponin I, on the other hand, is specific for cardiac muscle. However,
negative levels of CK-MB or troponin I do not rule out clinically relevant BCI,
because in many cardiac trauma patients muscle disintegration is not
significant enough to release detectable enzyme levels. Yet even a small area
of myocardial damage can cause dysrhythmias if it is in a critical location.
Echocardiography may be helpful in many ways in
BCI. It provides information about myocardial function (wall motion
abnormalities, increased end-diastolic wall thick-ness), cardiac structural
abnormalities (echo-dense areas on the ventricular walls, valve malfunction,
hemopericardium, intracardiac thrombi), cardiac preload (end-diastolic area),
systolic cardiac function (fractional ventricular area change), and air
embolism (air bubbles in the cardiac chambers, patent foramen ovale).
Echocardiography can help not only with the diagnosis of BCI but also in
hemodynamic manage-ment. TEE is a much more valuable monitor than TTE, whose
usefulness is limited by mechanical ventilation, pleu-ral effusion,
pneumothorax, and difficulty in placing the patient in left lateral decubitus
position. Furthermore, myocardial contusion, hemopericardium, valvular lesions,
hemomediastinum, and aortic rupture are more likely to be recognized with TEE
than with TTE. Nevertheless, it is important to eliminate esophageal injury before
attempting TEE in chest trauma patients. However, dysrhythmias, unex-plained
hypotension, and/or heart failure are definite indica-tions for TTE or TEE.
Perioperative Management
Depending on its type and extent, BCI can
increase surgi-cal risk. Although most patients with cardiac chamber
perforation do not reach the hospital, some, especially those with atrial
lesions, may reach the operating room. Thus pre-operative information about the
nature of injury is impor-tant. Some retrospective reports have documented an
increased incidence of intraoperative dysrhythmias and hypotension in patients
with preoperatively diagnosed myocardial contusion. It is not entirely clear
whether these occurrences were due to the myocardial injury itself or to the
complications of associated injuries. Nevertheless, the combination of atrial
fibrillation, old age, and aortic rupture with myocardial contusion appears to
increase perioperative mortality. The duration of complications is also a
relevant issue for the anesthesiologist, since many trauma patients present
days to months after injury. In the vast majority of patients, dysrhythmias
last no more than a few days. Ventricular wall motion abnormalities may persist
for up to a year, but any increased risk of perioperative complications appears
to last for no more than 1 month. An intracardiac thrombus, a well-known
complication of myocardial contu-sion, may be present more than 1 year after
injury, further emphasizing the need for preoperative echocardiography even
well after the accident.
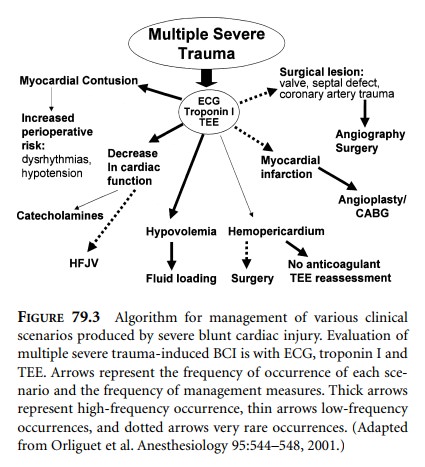
The clinical presentation of patients with BCI varies; sometimes more than one compromising condition may be present in the same patient. Orliguet et al. (2001) proposed an algorithm describing the management principles for each of these scenarios (Figure 79.3). Dysrhythmias appear to respond readily to antiarrhythmic agents. Hypotension may be caused by hypovolemia, pump failure, or both. Fluid loading, with monitoring of heart function by echocardiography or right heart catheterization, will improve hypovolemia. Pump failure is usually caused by right ventricular dysfunction exacerbated by increased pul-monary vascular resistance due to pulmonary contusion, aspiration of gastric contents, or ARDS. An initial right ventricular free wall dilatation may be followed by leftward ventricular septal shift, which alters the geometry and compliance of the left ventricle increasing left ventricular filling pressure and decreasing cardiac output. This should by no means be a signal to decrease fluid loading. On the contrary, volume replacement should continue with concomitant use of inotropes and pulmonary vasodilators. Positive-pressure ventilation may also be adjusted to mini-mize intrathoracic pressure and thus right ventricular afterload. High-frequency jet ventilation with its relatively low mean airway pressure may be beneficial in these circumstances. As shown in the algorithm, hemoperi-cardium is treated by drainage, either surgically or by placement of a large-bore catheter with echocardiographic guidance. Anticoagulants, if used, should be stopped. Myocardial infarction and valvular, septal, and coronary vascular injuries are treated in the same way as they would be in the absence of trauma. Of course, severe trauma may dictate surgery before angioplasty, coronary artery bypass, or repair of injured cardiac valves or a septal defect.
Very rarely pump failure may be unresponsive to
pharma-cologic measures and requires cardiac assistance. The ventricular
balloon pump has been used in these rare cases of BCI. However, in most
patients dysfunction originates from the right ventricle. Thus, if assistance
is needed, biventricular assist devices would probably be preferable.
Related Topics