Chapter: Medical Surgical Nursing: Terrorism, Mass Casualty, and Disaster Nursing
Weapons of Terror: Chemical Weapons
Chemical Weapons
Agents that may
potentially be used in chemical warfare
are overt agents in that the effects are more apparent and occur more quickly
than those caused by biological weapons. Agents are avail-able and well-known,
result in major mortality and morbidity,and cause panic and social disruption.
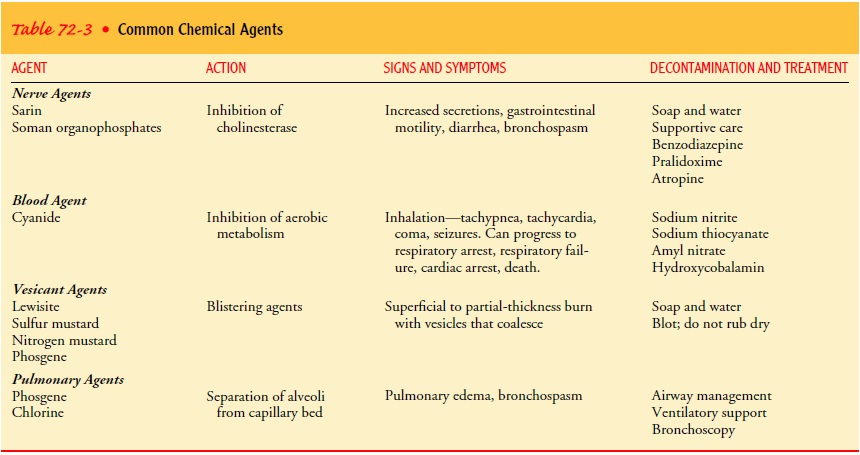
There are many agents, including those that affect nerves (sarin, soman), those
that affect blood (cyanide), those that are vesicants (lewisite, nitrogen and
sulfur mustard, phosgene), heavy metals (arsenic, lead), volatile toxins
(benzene, chloroform), pulmonary agents (chlorine), and corrosive acids (nitric
acid, sulfuric acid) (Table 72-3). Chlorine, phosgene, and cyanide are widely
used in industry and therefore are readily available.
CHARACTERISTICS OF CHEMICALS
Volatility.
Volatility is the tendency for a chemical to become
avapor. The most volatile agents are phosgene and cyanide. Most chemicals are
heavier than air, except for hydrogen cyanide. Therefore, in the presence of
most chemicals, the victim should stand up to avoid heavy exposure (because the
chemical will sink toward the floor or ground).
Persistence.
Persistence means that the chemical is less likely tovaporize and disperse. More volatile chemicals do not evaporate very quickly. Most industrial chemicals are not very persistent.
Weaponized agents
(chemicals developed as weapons by the mil-itary) are more likely than
industrial chemicals to penetrate and cause secondary exposure as well.
Toxicity.
Toxicity is the potential of an agent to cause
injury tothe body. The median lethal dose (LD50) is the amount of the chemical
that will cause death in 50% of those who are exposed. The median effective
dose (ED50) is the amount of the chemical that will cause signs and symptoms in
50% of those who are ex-posed. The concentration time (CT) is the concentration
released multiplied by the time exposed (mg/min). For example, if 1000 mg of a
chemical were released and the time of exposure to this amount of chemical was
10 minutes, then the concentration time would be 10,000 mg/min.
Latency.Latency is the time from absorption to the
appearanceof symptoms. Sulfur mustards and pulmonary agents have the longest
latency, whereas vesicants, nerve agents, and cyanide pro-duce symptoms within
seconds.
LIMITING EXPOSURE
Evacuation is essential,
as are removal of clothing and decontam-ination as close to the scene as
possible and before transport of the person exposed. Soap and water are
effective means of deconta-mination in most cases. Staff involved in
decontamination efforts must wear PPE and contain the runoff after
decontamination procedures.
VESICANTS
Vesicants are chemicals
that cause blistering and result in burning, conjunctivitis, bronchitis, pneumonia,
hematopoietic suppres-sion, and death. Examples of vesicants include lewisite,
phosgene, nitrogen mustard, and sulfur mustard. In World War I and in the
Iran–Iraq conflict of 1980–1988, vesicants were used to disable the opponent.
Vesicants were the primary incapacitating agents, resulting in minimal (less
than 5%) death but large numbers of injured (Brennan, Waeckerle, Sharp, &
Lillibridge, 1999). Liquid sulfur mustard was the most frequently used vesicant
in these conflicts. It is an oily liquid with a garlic odor, has a long latent
period, and penetrates the skin if not rapidly removed. The skin damage is
irreversible but is seldom fatal (2% to 3% mortality).
The initial presentation
after exposure to a vesicant is similar to that of a large superficial to
partial-thickness burn in the warm and moist areas of the body (ie, perineum,
axillae, antecubital spaces). There is stinging and erythema for approximately
24 hours, followed by pruritus, painful burning, and small vesicle formation
after 2 to 18 hours. These vesicles can coalesce into large, fluid-filled
bullae. Lewisite and phosgene result in immediate pain after exposure. Tissue
damage occurs within minutes.
If the eye is exposed, there is pain, photophobia,
lacrimation, and decreased vision. This progresses to conjunctivitis,
blepharo-spasm, corneal ulcer, and corneal edema.
Respiratory effects are more serious and often are the
cause of mortality with vesicant exposure. Purulent fibrinous pseudo-membrane
discharge leads to obstruction of the airways. Gastro-intestinal exposure
includes nausea and vomiting, leukopenia, and upper gastrointestinal bleeding.
Appropriate
decontamination includes soap and water. Scrub-bing and the use of hypochlorite
solutions should be avoided, because they increase penetration. Once the
substance has pene-trated, it cannot be removed. Eye exposure requires copious
irri-gation. For respiratory exposure, intubation and bronchoscopy to remove
necrotic tissue are essential. With lewisite exposure, dimercaprol (BAL in oil)
is administered intravenously for systemic toxicity and topically
for skin lesions. All persons with sul-fur mustard exposures should be
monitored for 24 hours for de-layed (latent) effects.
NERVE AGENTS
The most toxic agents in
existence are the nerve agents such as sarin, soman, tabun, VX, and
organophosphates (pesticides). They are inexpensive, effective in small
quantities, and easily dis-persed. In the liquid form, nerve agents evaporate
into a colorless, odorless vapor. Organophosphates are similar in nature to the
nerve agents used in warfare and are readily available. Nerve agents can be
inhaled or absorbed percutaneously or subcuta-neously. These agents bond with
acetylcholinesterase, so that acetylcholine is not removed; the adverse result
is continuous stimulation (hyperstimulation) of the nerve endings. Carbamates,
which are insecticides originally extracted from the Calabar bean, are
derivatives of carbamic acid; they are nerve agents that specif-ically inhibit
acetylcholinesterase for several hours and then spontaneously become unbound
from the acetylcholinesterase. Organophosphates, however, require the formation
of new en-zyme (acetylcholinesterase) before function can be restored.
A very small drop of
agent is enough to result in sweating and twitching at the site of exposure. A
larger amount results in more systemic symptoms. Effects can begin anywhere
from 30 minutes up to 18 hours after exposure. The more common
organophos-phates and carbamates that are used in agriculture (sevin and
malathion) result in less severe symptoms than do those used in warfare.
Signs and symptoms of
nerve gas exposure are those of cholin-ergic crisis and include bilateral
miosis, visual disturbances, in-creased gastrointestinal motility, nausea and
vomiting, diarrhea, substernal spasm, indigestion, bradycardia and
atrioventricular block, bronchoconstriction, laryngeal spasm, weakness,
fascicu-lations, and incontinence. The patient must be examined in a dark area
to truly identify miosis. Neurologic responses include insomnia, forgetfulness,
impaired judgment, depression, and ir-ritability. A lethal dose results in loss
of consciousness, seizures, copious secretions, fasciculations, flaccid
muscles, and apnea.
Decontamination with
copious amounts of soap and water or saline solution for 8 to 20 minutes is
essential. The water is blot-ted, not wiped, off. Fresh 0.5% hypochlorite
solution can also be used. The airway is maintained, and suctioning is
frequently re-quired. One must be aware that plastic airway equipment will
absorb sarin gas, resulting in continued exposure to the agent.
Treatment.Intravenous atropine 2 to 4 mg is administered,
fol-lowed by 2 mg every 3 to 8 minutes for up to 24 hours of treat-ment.
Alternatively, intravenous atropine 1 to 2 mg/hr may be administered until
clear signs of anticholinergic activity have re-turned (decreased secretions,
tachycardia, and decreased gastro-intestinal motility). Another medication is
pralidoxime; which allows cholinesterase to become active against acetylcholine.
Pralidoxime 1 to 2 g in 100 to 150 mL of normal saline solution should be
administered over 15 to 30 minutes. Pralidoxime has no effect on secretions and
may have any of the following side effects: hypertension, tachycardia,
weakness, dizziness, blurred vision, and diplopia.
Diazepam (Valium) or other benzodiazepines should be
ad-ministered for seizures, to decrease fasciculations, and to alleviate
apprehension and agitation. The military provides all military personnel with
Mark I autoinjectors, which contain 2 mg atro-pine and 600 mg pralidoxime
chloride. Diazepam is administered by a partner.
BLOOD AGENTS
Blood agents have a
direct effect on cellular metabolism, result-ing in asphyxiation through
alterations in hemoglobin. Examples include hydrogen cyanide and cyanogen
chloride. Cyanide is an agent that has profound systemic effects. It is
commonly used in the mining of gold and silver and in the plastics and dye
indus-tries. In 1984, the Union Carbide pesticide plant in Bhopal, India,
released large amounts of cyanide in an industrial disaster, and hundreds of
deaths occurred.
A cyanide release is often associated with the odor of
bitter al-monds. In house fires, cyanide is released during the combustion of
plastics, rugs, silk, furniture, and other construction materials. There is a
significant correlation between blood cyanide and carbon monoxide levels in
fire victims, and most often the cause of death is cyanide.
Cyanide can be ingested, inhaled, or absorbed through the
skin and mucous membranes.
Cyanide is protein bound and inhibits aerobic metabolism,
leading to respiratory muscle failure, respiratory arrest, cardiac arrest, and
death. Inhalation of cyanide results in flushing, tachy-pnea, tachycardia,
nonspecific neurologic symptoms, stupor, coma, and seizure preceding
respiratory arrest.
Emergency Treatment.
Rapid administration of
the followingmedications is essential to the successful management of cyanide
exposure: amyl nitrate, sodium nitrite, and sodium thiosulfate. First, the
patient is intubated and placed on a ventilator. Next, amyl nitrate pearls are
crushed and placed in the ventilator reser-voir to induce methemoglobinemia.
Cyanide has a 20% to 25% higher affinity for methemoglobin than it does for
hemoglobin; it binds methemoglobin to form either cyanomethemoglobin or
sulfmethemoglobin. The cyanomethemoglobin is then detoxified in the liver by
the enzyme rhodanase. Next, sodium nitrite is ad-ministered intravenously, also
to induce the rapid formation of methemoglobin. Sodium thiosulfate is then
administered intra-venously; it has a higher affinity for cyanide than
methemoglobin does and stimulates the conversion of cyanide to sodium
thio-cyanate, which can be renally excreted. There are side effects of these
emergency medications: sodium nitrite can result in severe hypotension, and
thiocyanate can cause vomiting, psychosis, arthralgia, and myalgia.
The production of
methemoglobin is contraindicated in pa-tients with smoke inhalation, because
they already have decreased oxygen-carrying capacity secondary to the
carboxyhemoglobin produced by smoke inhalation. An alternative suggested
treat-ment for cyanide poisoning is hydroxycobalamin (vitamin B12a).
Hydroxyocobalamin binds cyanide to form cyanocobalamin (vitamin B12).
It must be administered intravenously in large doses. Administration of vitamin
B12a can result in transient pink discoloration of
mucous membranes, skin, and urine. In high doses, tachycardia and hypertension
can occur, but they usually resolve within 48 hours.
PULMONARY AGENTS
Pulmonary agents such as
phosgene and chlorine destroy the pul-monary membrane that separates the
alveolus from the capillary bed. Hence, the person exposed cannot release
carbon dioxide or acquire oxygen. Capillary leak results in fluid-filled
alveoli. Phos-gene and chlorine both vaporize, rapidly causing this pulmonary
injury. Phosgene has the odor of fresh-mown hay.
Signs and symptoms
include pulmonary edema with shortness of breath, especially during exertion.
Cough starts as a hackingcough followed by frothy
sputum production. A mask is the only protection required. Phosgene does not
injure the eyes.
Related Topics