Chapter: Modern Pharmacology with Clinical Applications: Diuretic Drugs
Uses of Diuretics
USES OF
DIURETICS
The ability of certain drugs
to increase both fluid and electrolyte loss has led to their use in the
clinical man-agement of fluid and electrolyte disorders, for example, edema. Regardless of the cause of the syndrome
associ-ated with edema, the common factor is almost invariably an increased
retention of NA+ . The aim of diuretic ther-apy is to enhance NA+
excretion, thereby promoting negative NA+ balance.This net NA+
(and fluid) loss leads to contraction of the overexpanded extracellular fluid
compartment.
Congestive Heart Failure
Diuretics may have
considerable value in reducing the edema associated with congestive heart
failure; how-ever, each patient must be evaluated individually, since diuresis
is not considered mandatory in all patients. Digitalis and salt restriction may
be sufficient to de-crease the associated symptoms of pulmonary conges-tion and
peripheral edema. In patients who require
a di-uretic as adjunctive therapy, the usual choice should be a thiazide or
thiazide-type diuretic rather than one of the loop diuretics (e.g., bumetanide
or furosemide). This is true
especially in mild congestive heart failure. The more efficacious compounds
probably should be re-served for those who fail to respond to one of the
thi-azides. A K+ -sparing diuretic also can be given with the
thiazide to maintain serum K+ levels, which might oth-erwise be
depleted. Hypokalemia predisposes patients to digitalis intoxication.
Hypertension
The use of diuretic drugs,
either alone or in combination with other agents, in the management of mild to
mod-erate hypertension is frequent. Diuresis and restriction of salt intake are
often sufficient for all hypertensive pa-tients except those with severe,
malignant, or compli-cated hypertension. The mechanisms by which the di-uretics
lower arterial pressure are not precisely known, although it is thought that
the initial response is due to a reduction of plasma volume with a consequently
di-minished cardiac output. However, after a few weeks, the initial degree of
extracellular volume reduction is not maintained, probably owing to a gradual
increase in aldosterone production (i.e., increased NA+ retention
and K+ loss). Nonetheless, the antihypertensive effect is sustained.
Although the arterial
pressure in hypertensive pa-tients is related to intravascular volume, the
changes in plasma volume are primarily caused by alterations in to-tal body NA+
. Strict dietary NA+ restriction
can lower arte-rial pressure in hypertensive patients, whereas a large NA+
intake will reverse the hypotensive effects of thiazide di-uretics. It
appears quite plausible that all of the hypoten-sive effects of the diuretics
can be attributed to some as-pect of NA+ depletion, that is, either
directly on extracellular fluid volume or perhaps indirectly through the
effects of NA+ loss on autonomic nervous function (e.g., diminished
norepinephrine storage capacity in sym-pathetic nerves) or vascular smooth
muscle reactivity.
Diuretics are frequently used
in combination with other antihypertensive agents. The appropriateness of this
combination becomes even more apparent when it is realized that nondiuretic
antihypertensives (e.g., hy-dralazine or diazoxide) produce some increase in
plasma volume that if not corrected, would lead to an eventual decrease in
their activity .
Hepatic Ascites
Cirrhosis and other liver
diseases may result in the for-mation of excessive amounts of fluid in the
abdomen (ascites). The primary causes
of ascites are usually ele-vation of pressure in the portal vein and a
decreased amount of hepatic plasma protein production. Both fac-tors tend to
reduce the ability of the vascular compart-ment to retain fluid. The resultant
ascites may con-tribute to decreased appetite and respiratory difficulties,
among other symptoms. When these symp-toms are present, careful reduction in
the fluid volume through the use of diuretics is desirable.
Since patients with cirrhosis
vary widely in their re-sponse to diuretics, conservative initial diuretic
therapy is called for. The mainstay of
treatment, however, remains restriction
of dietary NA+ . A common finding in patients with cirrhosis is decreased glomerular
filtration, despite the increase in total blood volume caused by the exten-sive
pooling of blood in the splanchnic vessels. Diminished renal perfusion leads to
increased aldos-terone secretion, which in turn increases NA+ retention
and K+ loss. Thus, in addition to diuretics, most patients require K+
supplementation. The thiazides
remain the drugs of first choice. The
use of a high-ceiling drug, such as
furosemide, leads more frequently to such complica-tions as hypokalemia,
hyponatremia, and azotemia. K+ - sparing diuretics may be useful
adjunctive (but not sole) agents if extensive hypokalemia is present.
Pulmonary Edema
The usual cause of pulmonary
edema is acute left ven-tricular failure. The sequelae of events after left
heart failure roughly follow the pattern of reduced stroke vol-ume, leading to
increased end-systolic and diastolic vol-ume, which elevates left ventricular
end-diastolic pres-sure. Pressure then increases in the left atrium, pul-monary
vein, and finally in the pulmonary capillaries. Elevated pressure in the
pulmonary capillaries results in the passing of more fluid into the pulmonary
intersti-tial space, and this compromises gas exchange, dimin-ishes total lung
gas volume, and increases airway resist-ance. With acute pulmonary edema of cardiac origin, the traditional treatment has included
administration of the efficacious, rapidly acting loop diuretics. These
agents, given parenterally, can
reduce total blood volume rap-idly and thus may help to prevent recurrence of
pul-monary congestion. The value of immediate and vigor-ous use of the loop
diuretics has been questioned. The problems of excessive fluid and K+ loss
indicate a con-servative approach to diuresis even in this medical emergency.
Increased Intracranial Pressure
A rise in intracranial
pressure results in the appearance of a number of symptoms, including headache,
vomit-ing, edema of the optic discs, changes in vital signs, and possibly
death. Dehydrating measures, including the use of diuretics, can help lower the
pressure, particularly if the elevated intracranial pressure is of a
nontraumatic origin. The parenteral
administration of a hypertonic so-lution of one of the osmotic diuretics, urea
or mannitol, can relieve the pressure through its osmotic effects. The oral administration of glycerol also
has been used in neurosurgical procedures when increases in intracranial
pressure are anticipated.
Renal Edema
Nephrotic Syndrome
Nephrotic syndrome is
characterized by proteinuria and edema due to some form of glomerulonephritis.
The re-sulting fall in plasma protein concentration decreases vascular volume, which
leads to diminished renal blood flow. This in turn causes secondary
aldosteronism char-acterized by NA+ and water retention and K+ depletion.
Rigid control of dietary NA+ is essential. Therapy of the nephrotic
syndrome using a thiazide (possibly with a K+ - sparing diuretic) to
control the secondary aldosteronism, is a useful initial approach to treatment.
Since nephrotic edema is
frequently more difficult to control than car-diac edema, it may be necessary
to switch to a loop di-uretic (and spironolactone) to obtain adequate diuresis.
Chronic Renal Failure
The loop diuretics are usually required in treating chronic renal
failure, since drugs with lesser intrinsic ac-tivity are not sufficiently
effective when tubular function has
been compromised greatly. Larger than normal amounts of furosemide are
frequently employed, and thus it is especially important to monitor the patient
for excessive volume depletion. Intermittent therapy may be the best approach.
Acute Renal Failure
The principal rationale for the
use of diuretics in acute renal failure is to prevent complete renal shutdown.
Whether renal failure is caused by some underlying dis-ease or by drug-induced
renal toxicity, the continued production of even a small amount of urine is
probably important in reducing further kidney tubular damage. Most commonly employed are the osmotic
diuretics, with intravenous mannitol generally being the agent of choice. Osmotic
diuresis is possible only if glomerular damage, tubular damage, or both have
not progressed too far.
Premenstrual Edema and Edema of Pregnancy
Many women retain fluid
during pregnancy and during the last days of the menstrual cycle. Breast
fullness and subcutaneous swelling or puffiness are the most com-monly observed
symptoms; they are largely the result of elevated circulating hormone levels in
the blood. Estrogens possess some mineralocorticoid activity, and thus, when
present in relatively high concentrations, may produce some expansion of the
extracellular fluid compartment. Excessive premenstrual edema fre-quently
responds well to thiazide therapy. Recent
expe-rience has diminished enthusiasm for use of any diuret-ics in pregnant
women. Since the edema of pregnancy is
frequently well tolerated, concerns of compromised uteroplacental
perfusion, possible ineffectiveness of di-uretics in preeclampsia, and the risk
of adverse effects of diuretics on the baby (e.g., thiazides can both cross the
placental barrier and appear in breast milk, produc-ing electrolyte
disturbances and thrombocytopenia in newborns) have led to diminished routine
use of these agents in pregnancy.
Resistance to Diuretic Administration
Since the effectiveness of
many diuretics ultimately de-pends on establishing a negative NA+ balance
to mobi-lize edema fluid, restriction of
dietary NA+ intake is gen-erally an essential part of diuretic
therapy. Therefore, one cause of
therapeutic failure or apparent patient refrac-toriness to diuretics could be
the patient’s continued in-gestion of large quantities of NaC1.
Some of the older diuretic
drugs were self-limiting; that is, prolonged administration resulted in a
gradual diminution of their effectiveness. This problem was cor-rected through
the use of intermittent diuretic therapy. Such a program of several days of
diuresis followed by several days of drug withdrawal delayed refractoriness to
the drug by preventing excessive disturbances in body electrolyte composition.
Many diuretics (e.g., thiazides and loop diuretics) must reach the
tubular lumen before they begin to be ef-fective. Because these compounds are
organic acids and are bound to plasma
proteins, they reach the lu-minal fluid by secretion. Any disease condition or
drug that impairs secretion will
affect the access of the di-uretics to the luminal fluid and hence to their
ultimate site of action (e.g., distal tubule or ascending loop). For example,
renal dysfunction may lead to a buildup of endogenous
organic acids that decrease drug secretion and thereby alter the patient’s expected response to the diuretic.
Patients with azotemia frequently require large doses of organic acid diuretics
to achieve a satis-factory response. The concomitant administration of other
drugs that are substrates for the organic acid se-cretory system (e.g.,
probenecid or penicillin) may re-sult in an apparent resistance to diuretic
action. It should now be obvious that in
addition to disease and electrolyte
imbalances, the pharmacodynamic handling of the diuretics themselves may be a
factor in diuretic resistance.
Although most individuals
respond well to the usual doses of loop diuretics, a small number of pa-tients
are refractory to these drugs. These patients may be vulnerable to ototoxicity
or other adverse effects if larger amounts of the diuretic are employed.
Compensatory proximal tubular sodium absorption may contribute to or be
responsible for the resistance to loop diuretics. Combinations of diuretics may
be used as an alternative approach to treating diuretic re-sistance once it has
been verified that satisfactory NA+ restriction is being followed
and that the drug is being adequately absorbed. Administration of a carbonic
an-hydrase inhibitor may be sufficient to enhance NA+ de-livery to
thick ascending limbs, where its reabsorption can be blocked by loop diuretics.
Alternatively, thiazide diuretics may be combined with the loop diuretic to
limit absorption by distal convoluted tubules. The thi-azidelike diuretic
metolazone, which has some proxi-mal tubule effects unrelated to carbonic
anhydrase, ap-pears to be the most effective of the thiazide and thiazidelike
drugs in this regard.
Excessive Diuresis
Excessively vigorous diuresis
may lead to intravascular dehydration before removal of edema fluid from the
rest of the extracellular compartment. This is especially dangerous if the
patient has significant liver or kidney disease. Once the initial correction of fluid and elec-trolyte derangement has
been achieved, the effect sought is maintenance of homeostasis, not
dehydration. Drug dosage,
frequency of administration, and NA+ intake should be adjusted to
achieve homeostasis.
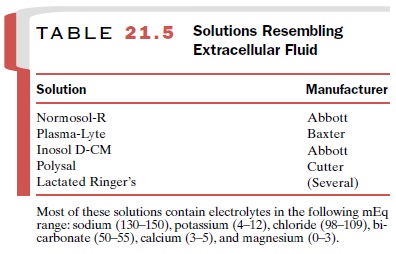
If diuresis has been too
vigorous, as may occur after injudicious use of loop diuretics, or if extensive
fluid and electrolyte loss has occurred following severe diarrhea or vomiting,
replacement therapy may be required. A number of available solutions resemble
extracellular fluid and are useful for the repair of water and elec-trolyte
deficits (Table 21-5).
Since the 1950s, diuretic
therapy has changed dra-matically. Earlier, the major diuretics were
acid-forming salts, xanthines, organomercurial compounds, and carbonic
anhydrase inhibitors. Either because of toxicity or lack of efficacy, these
agents are rarely if ever used.
Related Topics