Chapter: Modern Pharmacology with Clinical Applications: Diuretic Drugs
Body Water and Electrolyte Metabolism
BODY WATER AND
ELECTROLYTE METABOLISM
Body fluids are partitioned
between the intracellular fluids (ICF), which constitute two-thirds of total
body water, and extracellular fluids (ECF), which constitute one-third of total
body water. The ECF consists of plasma and interstitial fluid plus lymph. The
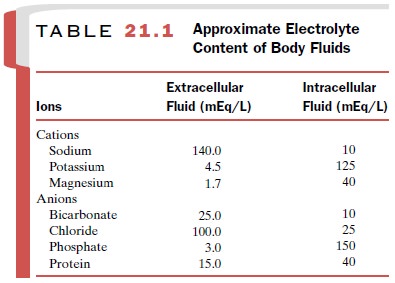
ionic com-position differs substantially between ECF and ICF (Table 21.1).
Sodium is the primary cation in ECF, whereas potassium is the principal
intracellular cation.
The concentrations and
distribution of electrolytes are not fixed, because cell membranes are permeant
to ions and to water. Movement of ions and water in and out of cells is
determined by the balance of thermody-namic forces, which are normally close to
equilibrium. Selective changes of ion concentrations cause move-ment of water
in or out of cells to compensate for these alterations. The kidneys are a major
site where changes in salt or water are sensed. The loss of fluids due to
ill-ness or disease may alter intracellular and extracellular electrolyte
concentrations, with attendant changes in fluid movement in or out of cells.
Changes of extracel-lular or intracellular ion concentrations, particularly for
potassium, sodium, and calcium, can have profound ef-fects on neuronal
excitability and contractility of the heart and other muscles.
Glomerular Filtration
Urine formation begins with the ultrafiltration of blood at the glomerulus. None of the available diuretics exerts its effects by altering the rate of glomerular filtration. Some agents, discussed later, reduce the glomerular fil-tration rate (GFR). However, this generally is an unde-sired or adverse reaction. Furthermore, at reduced GFRs, the delivery of sodium to the loop of Henle and the distal convoluted tubule, where the most efficacious classes of diuretics act, may be sufficiently compromised to reduce the action of the drugs.
Understanding the process of filtration is important to understanding the
pharmacokinetics of diuretic action because most of these agents exert their
inhibitory effect by blocking the entry of sodium from the urine into the cell.
Therefore, these diuretics have to be present at
sufficient concen-trations within the tubular fluid to exert their inhibitory
action on sodium transport. Most diuretics are variably bound to albumin and
therefore are only partially fil-tered. They gain access to the tubular fluid
by secretion into the proximal tubule (discussed later). In conditions of
hemorrhage or liver disease resulting in hypoalbu-minemia, the concentration of
albumin is reduced and the fraction of bound diuretic is altered. Although this
may suggest that more of the diuretic is unbound (or free) and filtered at the
glomerulus, this does not occur. The decrease in Starling forces, which govern
the rate of fluid filtration across the glomerular and other capillar-ies, now
results in greater entry of fluid into the intersti-tial space.
Most estimates of diuretic
binding to albumin as-sume that the protein itself is not altered as part of
the disease process. In renal failure, however, the number of binding sites on
the protein may change, which in turn affects the pharmacokinetics and dynamics
of the re-sponse to an administered diuretic. Another setting as-sociated with
diminished effective diuretic concentra-tions occurs in nephrotic syndrome. In this disease, protein escaping from the
glomerulus into the tubules binds the diuretic within the lumen. The bound drug
is unavailable to exert its inhibitory effect on sodium transport.
Tubular Reabsorption and Secretion
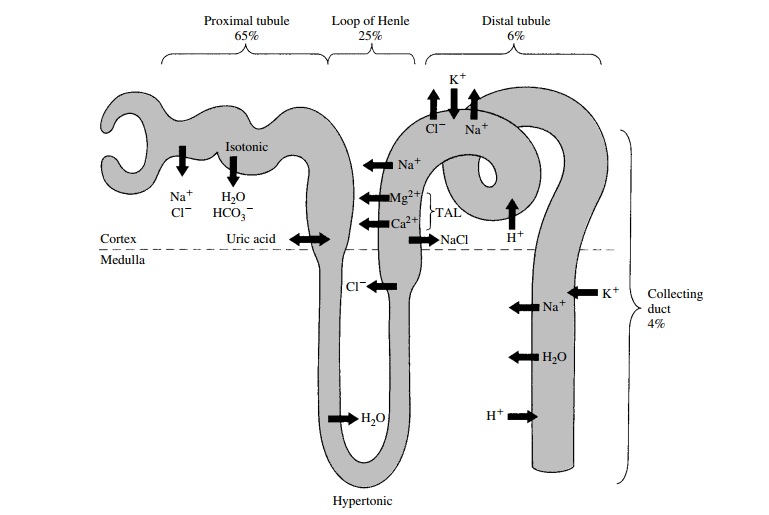
Two additional processes that participate in urine for-mation are reabsorption and secretion. Reabsorption defines movement of solute or water from the tubule lu-men to the blood, whereas secretion denotes transport from the blood to the tubule lumen. For many solutes, such as organic acids, transport proceeds in both direc-tions. Net transport is determined by the dominant flux. As described later, the tubular secretion of some di-uretics is critical for their action. The nephron sites where ions and organic solutes are transported are spa-tially separated. Figure 21.1 illustrates the various nephron segments, the primary sites of solute transport, and the magnitude of sodium reabsorption. In some in-stances, as with sodium, several transport mechanisms mediate its reabsorption. Importantly, each mechanism is spatially separated within different nephron seg-ments. This is important in understanding diuretic ac-tion, which is specific to particular sodium transport mechanisms. Furthermore, some common side effects caused by diuretics, such as potassium wasting, develop as a direct consequence of the mechanism and the par-ticular location of diuretic action at sites upstream from the distal nephron. The emphasis of the following sec-tions is on the tubular transport properties that affect or are influenced by diuretics.
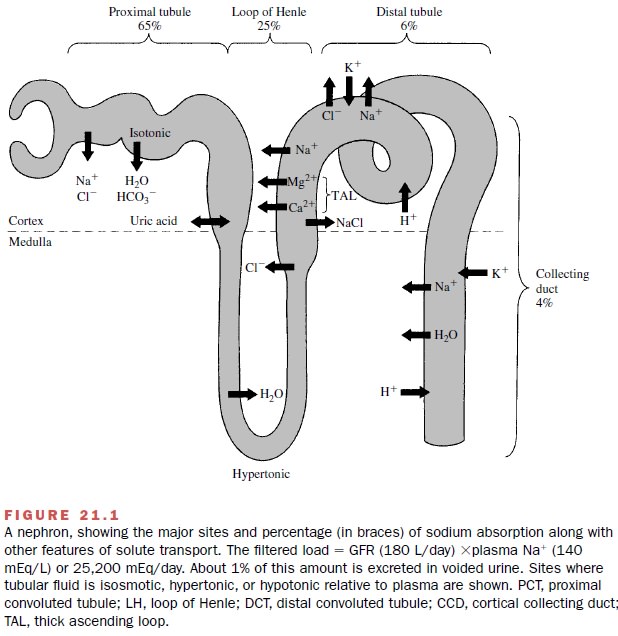
Proximal Tubule
The majority (two-thirds) of
filtered NA+ is reabsorbed by proximal tubules. A number of
transport mecha-nisms, including NA+ –H+ exchange, NA+
–phosphate co-transport, NA+ –glucose, NA+ –lactate, and NA+
–amino acid cotransport, participate in NA+ reabsorption. NA+
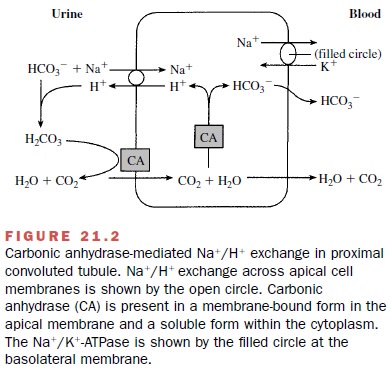
–H+ exchange is the primary
mechanism of NA+ transport in the proximal tubules (Fig. 21.2). NA+
and HCO3- enter
the proximal tubule after being filtered at the glomerulus. NA+ diffusion
from the lumen into the cell is coupled to the extrusion of a hydrogen ion into
the lumen. In the lumen, the H+ combines with HCO3-
to form carbonic acid (H2CO3), which in the presence of
the zinc metalloenzyme carbonic anhydrase
is rapidly converted to H2O and CO2. The CO2
generated in this reaction readily diffuses into proximal tubule cells, and the
process reverses. That is, the CO2 that was generated combines with
intracellular water and in the presence of cytoplasmic carbonic anhydrase forms
carbonic acid. The carbonic acid in turn is dehydrated to HCO3-
and H+ . The HCO3- is transported across the
basolateral membranes into the blood, while the H+ becomes
avail-able for another cycle of NA+ –H+ exchange. The net
re-sult of this process is the reabsorption of NA+ and HCO3-
. Carbonic anhydrase plays a pivotal role
both in the cytoplasm and in the
lumen in mediating NA+ –H+ ex-change and thus in some 40%
of total proximal NA+ and H2O absorption. If this
enzyme is inhibited, NA+ ab-sorption is slowed because of the
accumulation of H2CO3 in the lumen and the lack of H+
within the cell that can be exchanged for NA+ . Similarly, HCO3-
reab-sorption is reduced with a concomitant increase of HCO3-
excretion.
Several additional noteworthy features of proximal NA+ transport are relevant to diuretic action. First, since several transport proteins mediate proximal NA+ reab-sorption, no single diuretic would be expected to inhibit all these processes. Consequently, inhibition of any one mechanism leaves the others unaffected and able to continue to absorb the remaining NA+ .
Second, NA+ that escapes
proximal tubular transport is delivered to more distal nephron segments, where
compensatory reab-sorption reduces the impact of diminished upstream NA+
recovery. Hence, although most NA+
is reabsorbed by proximal tubules,
diuretics inhibiting its transport in this nephron segment have only a modest
effect in reduc-ing overall NA+ reabsorption.
Most of the K+ that is filtered at the glomerulus is
re-absorbed by proximal tubules. K+ appearing in the voided urine was secreted by distal and
terminal nephron segments (discussed later).
Another significant feature
of the proximal tubule is that it is the site of organic acid transport. This
is im-portant in understanding both the pharmacokinetics of many of the
diuretics, most of which are weak organic acids, and also certain of the side
effects induced by these drugs. For instance, uric acid, which is the end product
of purine metabolism in humans, is both reab-sorbed and secreted by the organic
acid transport path-way .
An important functional
characteristic of the proxi-mal tubule is that fluid reabsorption is isosmotic;
that is, proximal reabsorbed tubular fluid has the same osmotic concentration
as plasma. Solute and water are trans-ported in the same proportions as in the
plasma because of the high water permeability of the proximal tubule. Thus, the
total solute concentration of the fluid in the proximal convoluted tubule does
not change as the fluid moves toward the descending loop of Henle. The
corol-lary of this high water permeability is that unabsorbable or poorly
permeable solutes in the luminal fluid retard fluid absorption by proximal
tubules. This is an impor-tant consideration for understanding the actions
of os-motic diuretics.
Loop of Henle
Descending Thin Limb
The descending thin limb of
Henle’s loop begins at the end of the proximal straight tubule and continues
past the hairpin bend in Henle’s loop to the start of the thick ascending limb.
Descending thin limbs are virtually
de-void of NA+ –K+ –ATPase and therefore do not
participate in active sodium reabsorption. Moreover, the descending thin limb is highly impermeable to
sodium and urea. Although the descending thin limb is not a site of diuretic
action per se, its permeability contributes importantly to the action of
osmotic agents because of its high water permeability. The presence of
unabsorbable solute in the lumen retards water absorption and thereby
contributes to the osmotic diuresis. Furthermore, drugs and other compounds in
the tubular fluid are concentrated as a re-sult of the removal of water as the
descending thin limb of long-looped nephrons passes through the hypertonic
renal medulla. The elevation of drug concentrations for agents working at
downstream segments may aid in rais-ing the drug concentrations to the levels
necessary for di-uretic action. These elevated concentrations would not be
achieved in the systemic circulation. The selective in-crease in the
concentration of these drugs within the tu-bular fluid may account for the
relatively selective action of these compounds on the kidney, even though the
same sodium transport proteins are present in other tissues.
Thick Ascending Limb
The thick ascending limb is a major site of salt absorp-tion and a principal locus of action of an important group of diuretics. Approximately 25% of the filtered sodium is reabsorbed by the thick ascending limb of Henle’s loop. Sodium transport in this nephron segment is mediated by NA+ –K+ –2Cl- cotransport (Fig. 21.3). This transporter is present only on the apical, or urine, side of the tubule cells. Although K+ is taken up by the transporter, little net K+ reabsorption occurs in the thick ascending limb because much of the absorbed K is recycled across the apical cell membrane back into the urine.
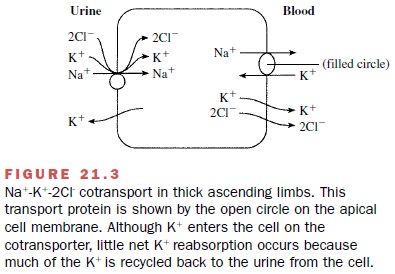
The recirculation of K+ is
important to the generation of the electropositive voltage within the lu-men,
which serves as a driving force for passive trans-port of NA+ , Ca++
, and Mg through the tight junctions joining adjacent cells. Hence, although K+ is transported by the NA+ –K+ –2Cl-
cotransporter, the primary solute ab-sorbed into the blood is NaCl.
Sodium reabsorption in thick
ascending limbs de-pends on the amount, or load, of salt delivered from
up-stream segments. The amount of sodium
reabsorbed by the thick ascending
limb increases as more is delivered. In situations such as severe volume
contraction, when abnormally large amounts of sodium are reabsorbed by proximal
tubules, little sodium reaches the thick as-cending limb. In this setting the
diuretic action of agents that block NA+ –K+ –2Cl-
cotransport is impaired. This is attributable to the reduction of sodium in the
tubular fluid of the thick ascending limb.
The reabsorption of NaCl by
the thick ascending limb is not accompanied by water because of the low
hydraulic permeability of this nephron segment. Consequently, the tubular fluid becomes dilute as it passes through the thick ascending limbs. This
process contributes to normal urinary
dilution. Moreover, when NA+ transport in thick ascending limbs is
inhibited, uri-nary dilution will diminish.
The thick ascending limb is
also an important site for the reabsorption of Ca++ and Mg . These
cations are mostly passively reabsorbed through the paracellu-lar pathway
between adjacent cells. The driving force for their transport is the
transepithelial voltage, which is established by the rate of NA+ reabsorption.
Thus, changes in voltage cause proportionate changes in the rate and magnitude
of Ca++ and Mg reabsorption.
Distal Convoluted Tubule
Sodium reabsorption continues
in the distal convoluted tubule, which accounts for some 6 to 8% of the trans-port
of sodium. The entry of NA+ across the apical cell membrane is
mediated by NA+ –Cl- cotransport (Fig. 21.4). This
protein is a distinct gene product that differs from the NA+ –K+
–2C1 cotransporter in thick ascend-ing limbs.
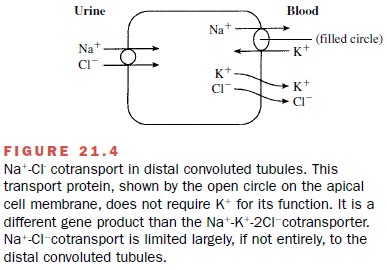
The permeability properties
of the distal convoluted tubule are regulated by antidiuretic hormone (ADH, or vasopressin). In hypotonic conditions,
ADH secretion by the posterior
pituitary is suppressed and the distal convoluted tubule is impermeant to
water. Conversely, in hypertonic or volume-contracted states, ADH is re-leased
by the posterior pituitary and increases the per-meability and water
reabsorption by the distal convo-luted tubule.
The distal convoluted tubule, along with the collect-ing duct, is an important site of K+ transport. The direction (reabsorptive or
secretory) and magnitude of K+ transport is governed by the
metabolic state of the indi-vidual, the amount and rate of NA+ and
fluid flow through the distal convoluted tubule, and the action of aldosterone.
As noted earlier, the main source of uri-nary K+ is tubular
secretion by distal convoluted tubules and collecting ducts. K+ secretion
also increases during alkalosis and with elevated dietary K+ intake.
Increases in the rate or amount of NA+
absorption or of the rate of fluid flow through the distal convoluted tubule
stimulate K+ secretion into the tubular fluid. These
ob-servations are especially important because they ac-count for the elevated K+
losses that attend the use of diuretics acting in more proximate
segments, such as thick ascending limbs and distal convoluted tubules.
In distal convoluted tubules,
calcium is transported by an active transport mechanism through rather than
between cells. Moreover, in distal convoluted tubules there is a reciprocal
relation between the direction and magnitude of calcium on NA+ transport.
As NA+ ab-sorption increases, calcium decreases, and conversely,
reductions of NA+ absorption are accompanied by ele-vated calcium
reabsorption. This interaction has impor-tant implications for diuretics acting
in the distal convo-luted tubule.
Collecting Ducts
The collecting ducts, which consist of cortical and medullary segments, reabsorb the final 5 to 7% of the filtered NA+ . The epithelium forming the collecting ducts consists of two distinct cell types: principal cells and intercalated cells. The relative preponderance of the two cell types varies along the length of the collecting duct and between nephron segments. Principal cells are responsible for the reabsorption of NA+ and the secre-tion of K+ (Fig. 21.5). NA+ enters the principal cell from the tubular fluid through a unique and highly selective epithelial NA+ channel, ENaC. Intercalated cells reab-sorb HCO3- and K+ and secrete H+ . Normally, H+ is se-creted into the urine and HCO3- is reabsorbed, while little net K+ transport occurs under K+ -replete conditions.
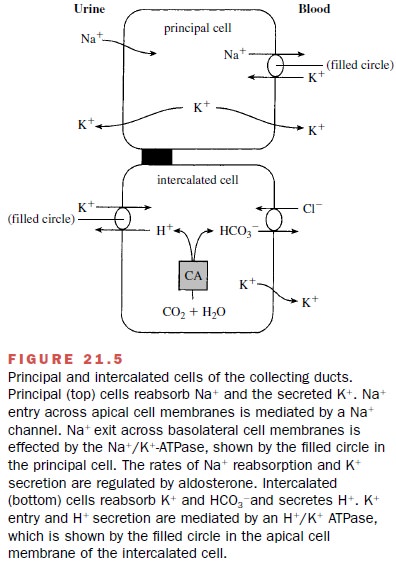
Aldosterone stimulates the
rates of NA+ reabsorp-tion and K+ secretion. This is
relevant to the action of spironolactone, a diuretic that is a competitive
inhibitor of aldosterone (discussed later). It is also pertinent be-cause
administration of diuretics can cause secondary hyperaldosteronism, which may
exaggerate the potas-sium wasting that is a consequence of the increased
de-livery of NA+ and enhanced flow through distal convo-luted
tubules and collecting ducts.
ADH can significantly modify
the total urine volume along with its solute concentration. In the absence of
ADH, the collecting ducts are essentially impermeable to water. Little fluid is
reabsorbed, and the final urine is dilute with respect to plasma. In other
words, the clear-ance of solute-free water (CH2O) is greater than the
os-molar clearance (Cosm). ADH increases water perme-ability,
allowing reabsorption of fluid from the tubules into the interstitium. The
driving force for water trans-port is the osmotic gradient between the
medullary in-terstitium and the tubular fluid. NaCl and urea are the two major
solutes accounting for the hypertonicity. The NaCl in the interstitium results
from the reabsorption of NA+ by thick ascending limbs. Thus, in the
absence of ADH, NA+ reabsorption contributes to medullary
inter-stitial hypertonicity, water abstraction from the collect-ing ducts, and
the formation of concentrated urine. Diuretics blocking NA+ reabsorption
by thick ascending limbs will therefore attenuate the formation of dilute urine
(CH2O) in hypotonic states when ADH is absent or low. Conversely, in
hypertonic conditions, when ADH levels are high and diuretics are blocking NA+
reabsorp-tion by the thick ascending limbs, the generation of con-centrated
urine is reduced, and Cosm is greater than CH2O.
Related Topics