Chapter: Pharmaceutical Drug Analysis: Ultraviolet and Absorption Methods
Ultraviolet and Absorption Methods: Assay Methods
ASSAY METHODS
1. METHODOLOGY
In general, when a radiation is made to pass through a
layer of a solution containing an absorbing pharmaceutical substance, a portion
of the radiation is absorbed by it, whereas the intensity of the radiation
emerging from the solution is always found to be less than the intensity of the
radiation entering it, Therefore, the quantum of the absorption is designated
in terms of the extinction E, that is represented by the following expression :
E = log 10 (Io/I)
where,
Io = Intensity of
radiation passing into the absorbing layer, and
I = Intensity of radiation
passing out of the absorbing layer.
Extinction is solely dependent upon the following two factors, namely :
(a)
Concentration of the absorbing substance present in the solution, and
(b) Thickness
of the absorbing layer taken for measurement.
Bearing in mind the ease in calculations and also the
convenience of reference, the extinction of a 1-cm layer of a 1% w/v solution
is usually recommended in most of the official compendia (i.e., USP ; BP ; EP : IP :) for many pharmaceutical substances and
is evaluated by the following expression :
E (1% ; 1-cm) = E/cl
where,
c = Concentration of the
absorbing substance represented as a percentage (w/v) ; and
l = Thickness of the absorbing
layer (cm).
It is however, pertinent to mention here that most pure
pharmaceutical substances possess a characteristic value of E (1% ; 1-cm) at a
specific wavelength in a given spectroscopic-grade solvent (UVASOL(R)-Merck).
This particular property is the basis for most assay methods included in pharmacopoeia
that are absolutely free from interfering materials, besides being utilized for
identifying substances.
In all other instances, the recommended tests specified
in pharmacopoeia and prescribed assay methods normally call for comparison
against Reference Substances (RS) to ensure measurements under conditions
identical for the substance under examination and the reference substance.
In actual practice, where a test or an assay recommends
the usage of a Reference Substance, the spectrophotometric measurements are
always performed first with the solution prepared from the Reference Substance
by the directions provided in the specific monograph and then with the
corresponding solution prepared from the substance under examination.
Nevertheless, the second measurement must be done immediately after the first,
by employing the same cell and the same instrumental parameters.
2. SPECTROPHOTOMETERS
Any appropriate spectrophotometer capable for measuring
both in the ultra-violet (UV) and visible range of the spectrum must
essentially consist of an optical system that should produce monochromatic
light in the range 190-780 nm and a suitable device for measuring the
extinction (E) precisely and accurately.
Besides, the two empty cuvettes (or cells) normally
employed for the solution under examination and the reference substance (RS)
should have exactly the same spectral features and characteristics.
Importantly, when a double bond recording instrument is being employed the
solvent cell is always placed in the reference beam.
3. PREPARATION OF SAMPLE
The pharmaceutical substance under examination is usually
dissolved in a spectroscopic grade UVASOL(R) SOLVENT. Particular
care must be taken to employ solvents free from contaminants absorbing in the
specific spectral region being used. In measuring the extinction of a solution
at a given wavelength, the extinction of the solvent cell and its contents must
not exceed 0.4 and should be preferably less than 0.2 when measured with
reference to air at the same wavelength. Particularly, the solvent in the
solvent cell should always be of the same purity, grade and batch as that
employed to prepare the respective solution and above all it must be free from
fluorescence at the wavelength of measurement.
Ethyl alcohol, methyl alcohol and cyclohexane (UVASOL(R)-Grade)
employed as solvents shall have an extinction, measured in a 1 cm cell at 240
nm with reference to water (spectroscopic grade), not exceeding 0.10.
4. MEASUREMENT OF EXTINCTION (E)
(a) Unless
otherwise prescribed, measure the extinction (E) or the absorbance (A), at the
prescribed wavelength using a path-length of 1 cm at 25 ± 1°C (IP) and at 20 ±
1°C (BP). All the measure-ments are normally performed with reference to the
solvent used to prepare the solution being examined, unless otherwise indicated
in the individual monograph.
(b) In the case
of an assay or a limit test where the extinction forms the basis for a
quantitative determination, a manually scanning instrument is employed
invariably. In tests for identification, a recording instrument is always
preferred ; besides, the concentration of the solution and the path-length are
specifically monitored. In case, the laid down conditions are not suitable for
a particular instrument, the thickness of the solution (i.e., path-length) may be varied without altering the concentration
of the solution,
(c) Each assay
of a pharmaceutical substance by UV-method specifies a wavelength at which
maximum absorption takes place which implies the maximum occurring either
precisely at or in the vicinity of the given wave length,
(d)
Pharmaceutical assays (i.e.,
quantitative determinations) are normally performed at wavelength above 235 nm,
(e) In case,
the measurements are specifically to be carried out at a wavelength between the
range 190-210 nm, the following extra and special precautions must be adhered
to rigidly, namely :
(i) Purging the
cell compartments with N2,
(ii) Making use
of only spectroscopic grade solvents e.g.,
UVASOL(R) (Merck), and
(iii) Making
use of cells that are absolutely transparent in the region 190-210 nm.
(f) The
requirements for light absorption in the official
compendia invariably apply to the dried, anhydrous, or solvent free
material in all such monographs in which standards for loss on drying, water or
solvent content are provided.
5. EXAMPLES
A few typical examples for the assay of pharmaceutical
substances by UV-spectrophotometric method are described below :
A. Amoxycillin Trihydrate
Materials Required : Amoxycillin trihydrate : 0.17 g ; 100-ml volumetric flask ; 2 ; buffer
solution pH 9.0 (Solution : I :
Boric acid and Potassium Chloride (0.2 M)-Dissolve 12.366 g of Boric acid and 14.911 g of KCl in DW and dilute with water to
1000 ml ; Solution : II NaOH (0.2 N) : Dissolve 8.0 g of NaOH in CO2-free
DW to produce 1000 ml ; Now, transfer 50 ml of solution I into a 200-ml
volumetric flask and add to it 20.8 ml of solution II, then add sufficient DW
to make up the volume to 200 ml) : 10 ml ; acetic anhydride-dioxan solution (add
1 ml of acetic anhydride to 50-ml of dioxan) : 1.0 ml ; imidazole-mercury
reagent (dissolve 8.25 g of recrystallized imidazole in 60 ml of DW and add 10
ml of 5N HCl. Stir the solution magnetically and, add dropwise, 10 ml of a
0.27% w/v solution of Hg2Cl2. Adjust the pH to 6.8 ± 0.05 with 5 N HCl (about 4.0 ml is needed) and add
sufficient DW to produce 100 ml) : 10.0 ml ;
Procedure : Weigh accurately about 0.17 g
of amoxycillin trihydrate and dissolve in sufficient DW to produce 500 ml. Now, transfer 10 ml of this solution into a 100 ml
volumetric flask, add 10 ml of buffer solution pH 9.0 followed by 1 ml of
acetic anhydride-dioxan solution, allow to stand for 5 minutes, and add
sufficient water to produce 100 ml. Pipette 2 ml of the resulting solution into
each of the two stoppered tubes. To tube 1 add 10 ml of imidazole-mercury
reagent, mix, stopper the tube and immerse it in a water-bath previously
maintained at 60 °C for exactly 25 minutes, with occasional swirling. Remove
the tube from the water-bath and cool rapidly to 20 °C (Solution-1). To tube 2
add 10 ml of DW and mix thoroughly (Solution-2). Immediately, measure the
extinctions of Solutions 1 and 2 at the maximum at about 325 nm, as detailed
above, employing as the blank a mixture of 2 ml of DW and 10 ml of imidazole-mercury
reagent for Solu-tion-1 and simply DW for Solution-2.
Calculations : The content of C16H19N3O5S
may be calculated from the difference between the extinctions of Solution-1 and that of Solution-2 and from the
difference obtained by repeating the operation using 0.17 g of amoxycillin trihydrate (RS), instead of the sample being
examined and the declared content of C16H19N3O5S
in the amoxycillin trihydrate (RS).
Cognate Assays : Ampicillin can also be assayed
by employing the above method using 0.15 g of the sample.
B. Folic Acid
Theory : Folic acid (I) undergoes
cleavage by reduction with Zn-Hg in acidic medium to yield p-aminobenzoylglutamic
acid (II). The primary aromatic amino group present in the latter is
subsequently diazotized in the usual manner and coupled in acidic solution with
N-(1-naphthyl)-ethylenediamine hydro-chloride in the absence of light
(caution). The colour thus produced has a maximum absorption at 550 nm and the
extinction (E) is consequently compared with a calibration curve obtained from p-aminobenzoic acid (PABA) that has been
duly diazotized and coupled exactly in the same fashion as the p-aminobenzoylglutamic acid.
The reaction involved is expressed by the following
equation :
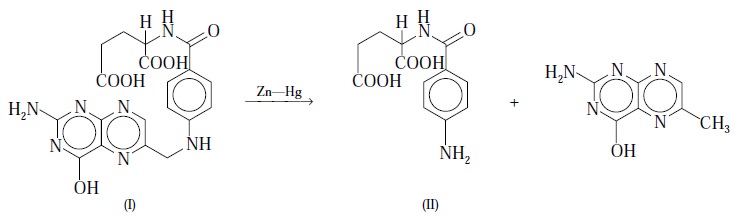
Note : In order to ensure that the extinctions recorded exclusively
refer to folic acid (I), and also that they do not necessarily include a
contribution from a free-primary-amino-aromatic-moiety obtained from a
decomposition product, a blank estimation is always performed with the
unreduced solution and an appropriate correction is applied. The colour thus
corresponds to a definite quantity of C16H19O6N7.
Thus, we have :
C7H7O2N = C19H19O6N7
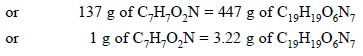
Materials Required : Folic acid : 0.05 g : 0.1 N NaOH : 100 ml ; 2 N HCl : 30 ml ; Zn-powder : 0.5 g ; sodium
nitrite solution (0.1% w/v in DW) : 5 ml ; ammonium sulphamate (0.5% w/v
in DW : 5 ml ; N-(1-naphthyl) ethylene-diamine hydrochloride solution (0.1 %
w/v in DW) : 5 ml ;
Procedure : Accurately weigh about 0.5 g,
dissolve in 50 ml of 0.1 N NaOH and add sufficient 0.1 N NaOH to produce 100 ml (Solution-1). To 3 ml add 20 ml of 2 N HCl
and dilute to 100 ml with DW. To 50 ml of this solution, add 0.5 g of zinc
powder, allow to stand in a dark place for 20 minutes with intermittent shaking
and filter, Dilute 10 ml of the filtrate to 25 ml with DW, add 5 ml of 2N HCl
and 5 ml of a 0.1% solution of sodium nitrite, mix and allow to stand for 2
minutes. Add 5 ml of a 0.5% w/v solution of ammonium sulphamate, mix and allow
to stand for 2 minutes. Now, add carefully 5 ml of a 0.1% solution of
N-(1-naphthyl) ethylene diamine hydrochloride, mix thoroughly and allow to
stand for 10 minutes. Add sufficient DW to produce 50 ml and measure the
extinction of the resulting solution at about 550 nm, as discussed earlier,
using as blank a solution prepared exactly in a similar manner but employing 25
ml of DW and beginning the procedure at ‘‘add 5 ml of 2 N HCl...’’
To a further portion of 30 ml of solution-1, add 20 ml of
2N HCl and sufficient DW to produce 100 ml. Mix 10 ml of this solution with 15
ml of DW and repeat the operations stated above beginning the procedure at
‘‘add 5 ml of 2 N HCl ...’’
Finally, substract 1/10th of the extinction of the
unreduced solution from that of the reduced solution and from the result thus
obtained calculate the amount of C19H19O6N7,
using the result obtained by repeating the operation using folic acid (RS)
instead of the substance being examined and the declared content of C19H19O6N7
in folic acid (RS).
C. Glyceryl Trinitrate Tablets
Theory : First and foremost the active
ingredient i.e., glyceryl
trinitrate is extracted completely from the
tables by shaking with glacial acetic acid. To an aliquot of the resulting
acetic acid solution an excess of phenoldisulphonic acid is added to produce a
yellow colour which is subsequently intensified by adding an excess of ammonia.
The following reactions take place :
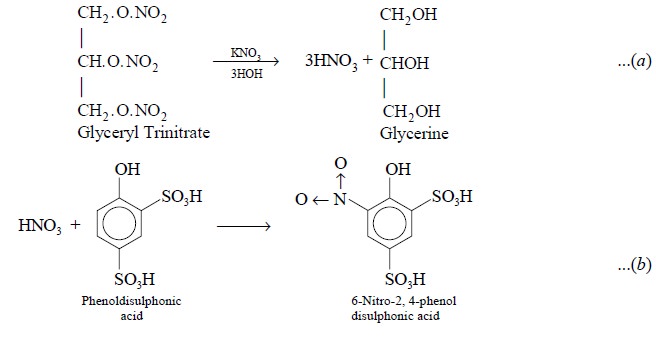
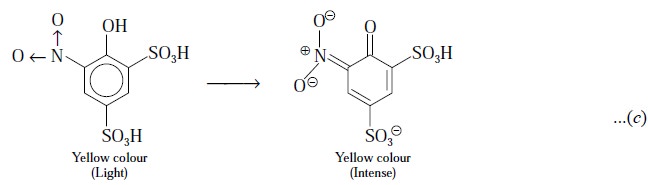
The standard substance in this assay is KNO3,
which conforms to the nitric acid released by acidolysis in the test solution.
Materials Required : Glyceryl trinitrate tablets :
20 ; glacial acetic acid (90% v/v) : 5 ml ;
phenoldisulphonic acid solution (heat 3 g of phenol with 20 ml of sulphuric
acid on a water-bath for 6 hours, and transfer the resulting liquid to a
stoppered vessel) : 2 ml ; strong ammonia solution ; 20 ml ; potassium nitrate
(previously dried at 105 °C) : 1 g ;
Procedure : Weigh and powder 20 tablets.
Now, weigh accurately a quantity of the powder equiva-lent to 0.5 mg of
glyceryl trinitrate, add 5 ml of glacial acetic acid, shake thoroughly for 1
hour and then centrifuge. To 2 ml of the supernatant liquid add 2 ml of
phenoldisulphonic acid solution and allow to stand for 15 minutes. Add 8 ml of
DW, make alkaline with strong ammonia solution, cool to about 20 °C, dilute to
20 ml with DW and filter. Finally, measure the extinction of a 1-cm layer of
the filtrate at 405 nm, as described earlier, employing as blank 2 ml of
glacial acetic acid, treated exactly in a similar fashion, begin-ning at ‘‘add
2 ml of phenoldisulphonic acid solution .........’’.
Dissolve 133.5 mg of potassium nitrate, in sufficient DW
to produce 100 ml ; to 10 ml add sufficient glacial acetic acid to produce 100
ml. Taking 2 ml of this solution, just repeat the assay beginning the procedure
at ‘‘add 2 ml of phenoldisulphonic acid solution......’’
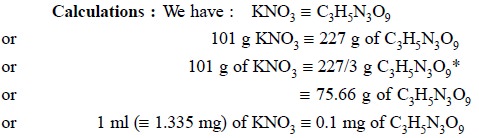
The content of C3H5N3O9
may be calculated from the values of the extinctions thus obtained. Each ml of
the potassium nitrate solution is equivalent to 0.1 mg of C3H5N3O9.
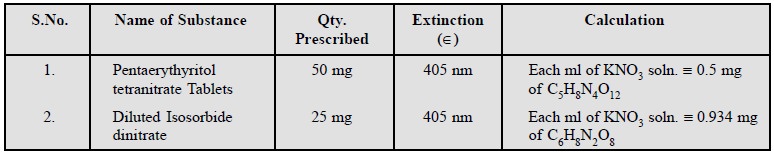
Cognate Assays : The following two
pharmaceutical products, namely : Pentaerythritol tetranitrate Tablets and Diluted Isosorbide
dinitrate are assayed by using a solution of phenoldisulphonic acid as detailed
below :
D. Stilboesterol
Theory : The assay of stilbonesterol is
exclusively based upon photochemical reactions whereby the trans-isomer firstly
gets converted into its corresponding cis-isomer
(Geometrical Isomerism) and then
followed by intramolecular
rearrangement therby causing ring closure as expressed in the equations :
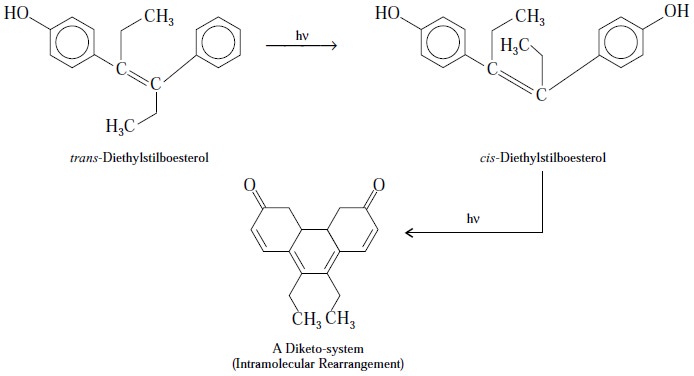
The highly conjugated diketo system obtained as a result
of irradiation of the stilbosterol solution placed in a closed
spectrophotometer cell for a duration of 10 minutes and exposed to a 15-watt
short-wave ultraviolet lamp. Ultimately the extinction is duly measured at 418
nm and compared with stilboesterol (RS) treated exactly in the same manner.
Materials Required : Stilbosterol : 20 mg ; ethyl
alcohol (absolute) : 250 ml ; dipotassium hydrogen phosphate solution (dissolve
1 g in 55 ml of DW) : 25 ml ;
Procedure : Weigh accurately about 20 mg
of stilbosterol in sufficient ethyl alcohol to produce 100 ml ; and dilute 10 ml of this solution to 100 ml with ethyl
alcohol. To 25 ml of the resulting solution add 25 ml of dispotassium hydrogen
phosphate solution, transfer a portion of the mixture to a 1-cm closed quartz
cell, place the cell 10 cm from a 15 watt short-wave UV-lamp, and subject it to
irradiation for 10 minutes. Now, measure the extinction of the irradiated
solution at the maximum at about 418 nm as described earlier.
Calculations : Calculate the content of C18H20O2 from the extinction obtained by
repeating the op-eration with stilbosterol (RS).
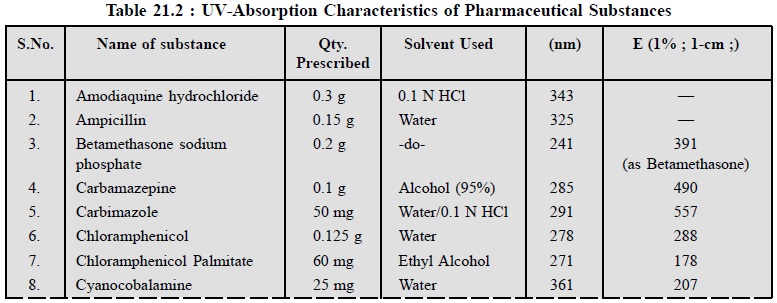
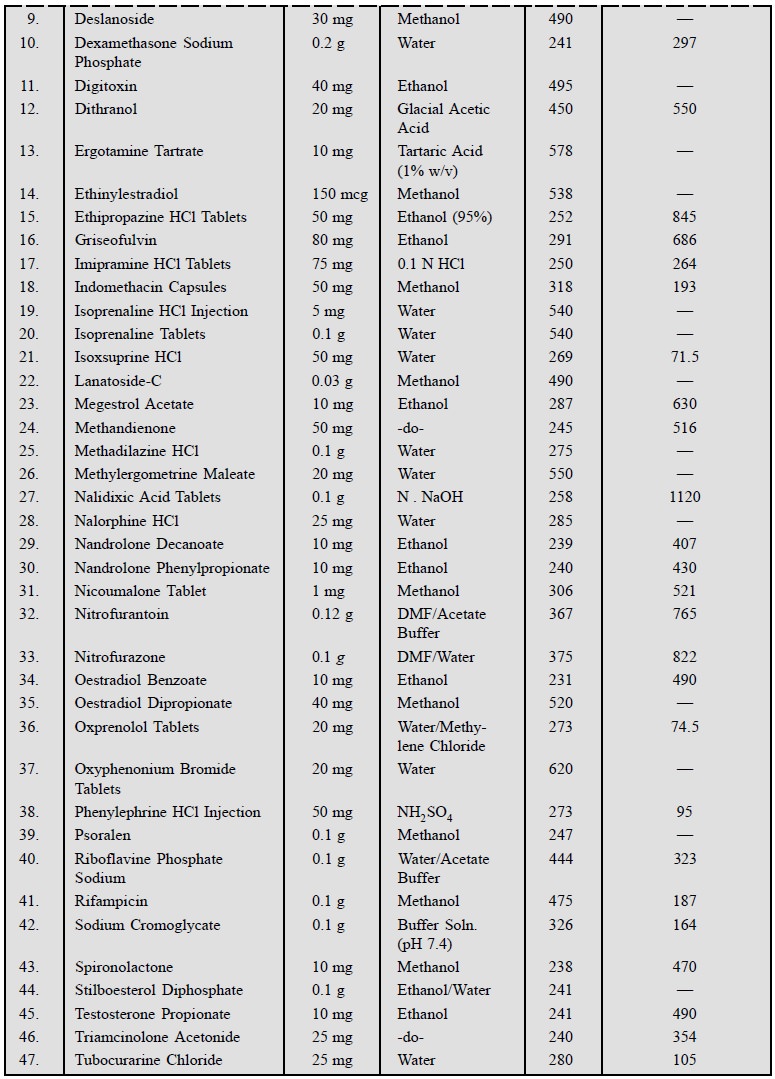
6. UV-ABSORPTION CHARACTERISTICS OF SOME OFFICIAL PHARMACEUTICAL SUB-STANCES
The ultra-violet absorption characteristics of a number
of official pharmaceutical substances have been duly provided in Table 21.2.
Related Topics