Chapter: Pharmaceutical Drug Analysis: Ultraviolet and Absorption Methods
Ultraviolet and Absorption Methods: Theory
THEORY
1. ELECTROMAGNETIC SPECTRUM
It has been established beyond any reasonable doubt that
the absorption and the emission of energy in the electromagnetic spectrum take place in distinct separate pockets or
photons. The relationship
existing between the energy of a photon and the frequency
matching its propagation may be expressed as follows :
E = hν ...(a)
where, E = Energy (in ergs),
v= Frequency (in cycles sec–
1), and
h = Universal constant termed as
Planck’s constant (6.6256 × 10 –
27 erg sec).
However, the relationship between wavelength and
frequency may be expressed as follows :
ν = c/λ ...(b)
where, λ = Wavelength (in cms),
c = Velocity of propagation of
radiant energy in vacuum (which is nothing but the speed of light in vacuum ; and is equivalent to 2.9979 ×
10 10 cm sec– 1).
The radiant power of a beam is designated by its
intensity of radiation, which in turn is directly proportional to the number of
photons per second that are propagated in the beam.
Monochromatic Beam : A beam that carries radiation
of only one distinctly separate wave length is known as monochromatic.
Polychromatic or Heterochromatic
: A
beam that carries radiation of several wavelengths is termed as polychromatic or heterochromatic.
2. SCHEMATIC REPRESENTATION OF ELECTROMAGNETIC SPECTRUM
Figure 21.1, provides a schematic representation of
electromagnetic spectrum, whereby the beam of a white light from an
incandescent solid (e.g., the
filament of an electric bulb consisting of numerous separate waves of different
wavelengths) is passed through a prism thereby giving rise to a continuous
spectrum wherein each colour corresponds to waves of a particular individual
wavelength.
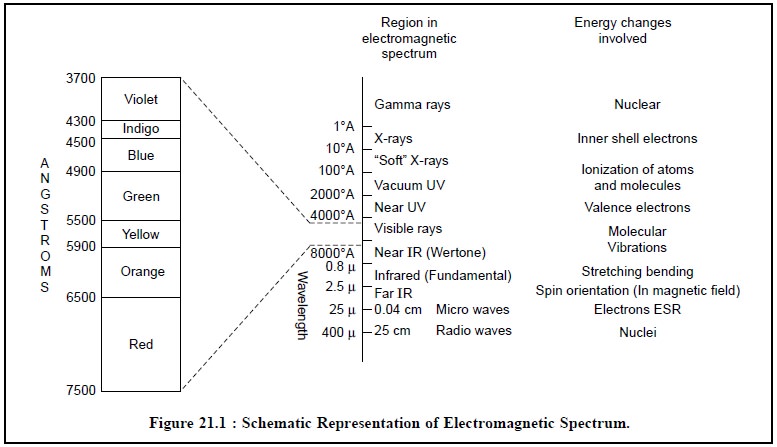
A few salient points from Figure 21.1 are enumerated
below :
(a) The visible
spectrum constitutes a small portion of the complete electromagnetic radiation
spec-trum that extends from the ultra-short wave gamma rays at one end to that
of the radio-waves at the other (400-700 nm),
(b) The wave
length scale is nonlinear,
(c) γ-Rays Region : Mossbauer Spectroscopy (due to absorption) and γ-Ray Spectroscopy (due to emission) are used as
analytical means.
(d) Inner-shell Electrons : X-Ray absorption spectroscopy (due to
absorption) and X-Ray Fluorescence spectroscopy (XRF) (due to emission) are
employed as analytical means.
(e) From Vacuum-UV to Infra-Red Region : UV-VIS, IR-spectroscopy, spectrophotometry,
atomic absorption spectroscopy (AAS)
(due to absorption) and atomic emission
spectroscopy (AES, ESS, ICP) ; atomic fluorescence spectroscopy (AFS)
(due to emission) are used as analytical techniques.
(f) Microwave Region : Microwave spectroscopy and electron
spin resonance (ESR) (due to absorption) are employed as analytical
methods.
(g) Radiowave Region : Nuclear Magnetic Resonance (NMR) (due to absorption) is used as
analytical method.
3. MOLAR ABSORPTIVITY
Usually, a molecule exists in the state of lowest energy the ground state. However, absorption of light of the right frequency
(in the UV-region) raises a molecule to an excited
state i.e., a state of higher energy. Considering the example
for ethylene two situations arise,
namely :
(a) Ground State : Here, both π
electrons are in the π orbital. This configuration
is designated as π2, where the superscript represents the number of
electrons in that orbital.
(b) Excited State : Here, an electron is in
the π orbital while the other in the π*
orbital (having an opposite spin). Thus, the resulting configuration ππ*
is obviously less stable due to the fact that :
(i) only one
electron helps to hold the atom together, and
(ii) the other
electron tends to force them apart.
The molar
absorptivity is mostly controlled by two
vital factors, namely :
(i)
polarity of the excited state, and (ii) probability of the electronic transition. So as to materialize
an interaction, a photon should evidently strike a molecule very closely within
the space of the molecular di-mensions. The probability of the electronic
transition, designated as ‘g’, shall
be responsible for the target hits that may ultimately lead to absorption.
However, the molar absorptivity may be expressed as follows :
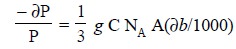 .......................(c)
.......................(c)
where, NA = Avogadro Number,
A = Cross-sectional target area*
1/3 = Statistical factor (to permit random orientation),
g = Probability of the
electronic transition
By inserting numerical constants and integration Eq. (c) we have :
log (Po/P) bC =
∈ = (0.87 × 10 20) g A ...(d)
where, ∈ = Molar absorptivity
Absorption with ∈ > 104 is
considered high-intensity absorption.
4. LAWS OF PHOTOMETRY
The ‘Laws of
Protometry’ has been discussed.
5. SPECTRAL PRESENTATION
Absorption spectra may be presented in a number of
fashions as depicted in Figure 21.2, namely :
(a) Wavelength Vs Absorbance,
(b) Wavelength Vs Molar Absorptivity, and
(c) Wavelength Vs Transmittance.
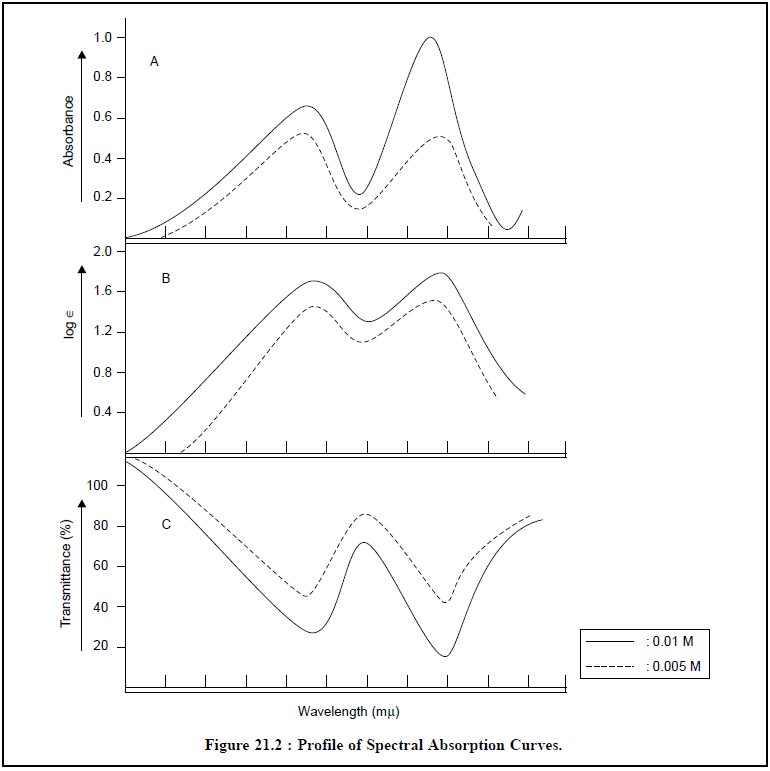
A few important features related to spectral presentation
are enumerated below :
(a)
In order to simplify the conversion of spectra in
qualitative identification the spectral data should be plotted either as log A
or as log ∈ Vs wavelength, thereby giving rise to the following expres-sion :
log A = log ∈ + log b + log c ...(e)
where, b = Cell-length, and
c = Sample
concentration.
From Eq. (e),
one may observe that the resulting curve is independent of both cell-length and
sample concentration,
(b) The
identity and nonconformity of sample may be ascertained by simply carrying out
the compari-son of spectral presentation both up or down the ordinate scale,
(c) In order to
obtain both reproducible and fairly consistent accurate plot the ordinate in
absorbance values must be plotted on graph paper having 1 mm equivalent to
0.005 absorbance,
(d) Most
importantly all relevant informations pertaining to : solvent employed,
concentrations used, the band pass and ultimately the Model/Make of the
Spectrophotometer,
(e) Choice of Solvents : For instance :
Water-common solvent for a number of inorganic
substances,
Ethanol (96% w/v)-good choice as fairly polar solvent,
Cyclohexane-common solvent for a number of aromatic
compounds.
6. STRUCTURAL FEATURES
While discussing the structural features special emphasis
shall be laid only to those molecules that are capable of absorption within the
wavelength region from 185 to 800 mµ.
A few salient
structural features are enumerated below :
(i) Compounds
having single bonds involving σ-valency electrons usually
display absorption spectra below 150 mµ. Such spectra will be
observed only in interaction with other types.
(ii) Excitation
help in promoting a p-orbital
electron into an antibonding σ orbit thereby giving rise to
an n → σ*
transition, for example : ethers, sulphides, amines, and alkyl halides.
(iii) Unshared p-electrons exist besides σ-electrons
in saturated compounds having covalent bonds and heteroatoms, for instance : N,
S, O, Cl, Br, I,
(iv)
Unsaturated compounds give rise to the absorption spectra by the displacement
of π-electrons.
(v) Molecules
that have single chromophores (i.e.,
absorbing groups)-normally undergo transitions almost very close to their
respective wavelengths,
(vi)
Interestingly, a molecule containing only a single chromophore of a particular
species shall absorb light of approximately the same wavelength as that of a
molecule having two or more insulated chromophores, however, the intensity of
the absorption shall be directly proportional to the number of the latter type
of chromophore present in the compound.
Examples :
(a) meta-orientation about an aromatic ring,
and
(b)
interposition of a single methylene (= CH2) moiety.
The above two instances are sufficient to insulate
chromophores from each other totally,
(vii) Hyperconjugation—is usually observed
when slight interaction takes place with alkyl radicals attached to
chromophores.
(vii)In fact, four
different types of absorption bands have so far gained cognizance in the
spectra of organic compounds, which are namely : K-bands ; R-bands ; B-bands ; and E-bands.
These bands will be discussed briefly here with regard to
the structural features.
(a) K-bands : They normally arise from π-π
structures and result from π → π* transitions.
These are invariably characterized by high molar
absorptivity.
Examples :
(i) A diene : C
= C—C = C to C+—C = C—C– ; where K-band is due to the
resonance transi-tion,
(ii) Vinyl
benzene or acetophenone : i.e.,
aromatic compounds having chromophoric substitu-tion.
(b) R-bands : They usually arise from n → π*
transitions. They seldom display very noticeable results in aliphatic
compounds, but marked and pronounced bathochromic shifts (i.e., shifting of absorption towards longer wavelengths—as in
extended open-chain-conjugated systems) do take place when—SH, —OH and —NH2
replace hydrogen atom in unsaturated groups. Thus, R-bands help in the
confirmation of a particular structure whereby additional bands are obtained by
appropriate modifications in the electronic-structure of the parent compound.
(c) B-bands : These are
rather weak-type of absorption bands.
They are characteristic of both heteroatomic and aromatic molecules and may
also consist of fine vibrational sub-bands.
(d) E-bands : They
usually result from oscillations of electrons in aromatic-ring systems,
(ix) Conjugated Systems :
It is quite evident that the conjugated systems might
fail to display the expected conjugated bands due to the following two reasons, namely :
(a) Orbitals of
adjacent multiple bonds are at right angles instead of being parallel, and
(b) Resonating
dipolar structures cannot be envisaged.
The resulting spectrum may seem to appear as a mere
superimposition of the spectra of the indi-vidual chromophoric groups.
Examples : Allene and ketene systems
Polyphenyls (e.g.,
m-terphenyl)
(x) Steric Hindrance : The attachment of
bulky functional entities to ring systems offering steric-hindrance may
ultimately prevent the coplanarity of two resonating structures either
completely or partially.
However, partial hindrance specifically leads to such
characteristic bands pertaining to those parts of conjugated system.
7. ABSORPTION OF RADIANT ENERGY BY MOLECULES
In reality, the molecules are as energetic as the modern
teenagers. They invariably rock, roll, twist, jerk, and bend, and if the music
is of the right rhythm, choice, and frequency, the electrons within the
molecule shall move from the ‘ground
state’ to the ‘excited state’.
Explicitly, the total energy in a molecule is the sum of
the energies associated with the translational, rotational, vibrational and
electronic motions of the molecule/or electrons/or nuclei in the molecule.
These four motion-related-energies
are briefly explained below :
(a) Transational Energy : It is associated
with the motion (velocity) of the molecule as a whole.
(b)
Rotational Energy : It is associated with the
overall rotation of the molecule.
(c) Vibrational Energy : It is associated
with the motion of atoms within the molecule.
(d) Electronic Energy : It is associated
with the motion of electrons arounds the nuclei.
Electrons generally found in the conjugated double bonds
invariably give rise to spectra in the UV and visible regions of the electromagnetic
spectrum.
It is pertinent to mention here that an excited electron
normally returns to the ground state in about 10–9 to 10–8
seconds. Consequently, energy must now be released to compensate for the energy
absorbed by the system. In actual practice however, the following three situations arise, namely :
Firstly, if the electron returns directly to the ground
state, the net effect would be evolution of heat. Secondly, if the electron
returns to the ground state by passing through a second excited state, the net outcome
would be release of energy in the form of heat and light.
Thirdly, if a large amount of energy is absorbed by
certain substances, bonds may be ruptured and thereby giving rise to altogether
new compounds.
For instance : ergosterol on being subjected to UV
radiation yields cholecalciferols which are, in fact, altogether new
substances.
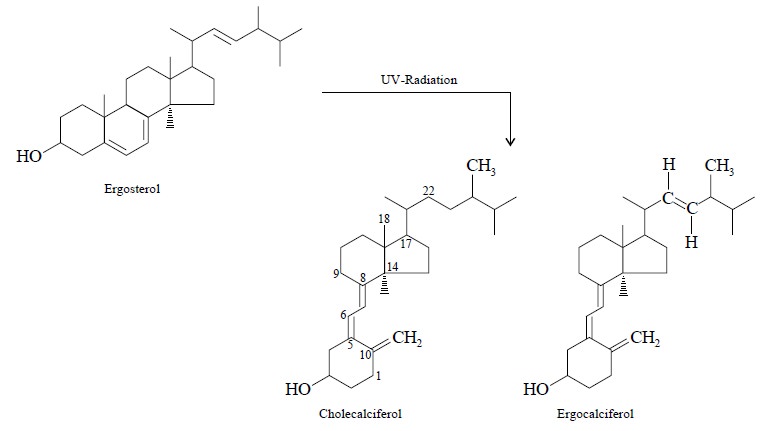
In general, the changes incurred are usually minimal and
for this very reason the UV-spectrophotometry is considered to be a
non-destructive method of analysis.
However, the relative energies due to electrons (d), vibration (c), and rotation (b) are
more or less in the order of 10,000 : 100 : 1 ; and the total energy for any
one state at any material time may be depicted by the following expression :
ETotal = EElectronic + EVibrational
+ ERotational
The diagrammatic representation of the potential energy
of a diatomic molecule showing :
(i) Potential
energy-nuclear separation curves, and
(ii)
Relationship between electronic transitions and
absorption curves ; is illustrated in Figure 21.3.
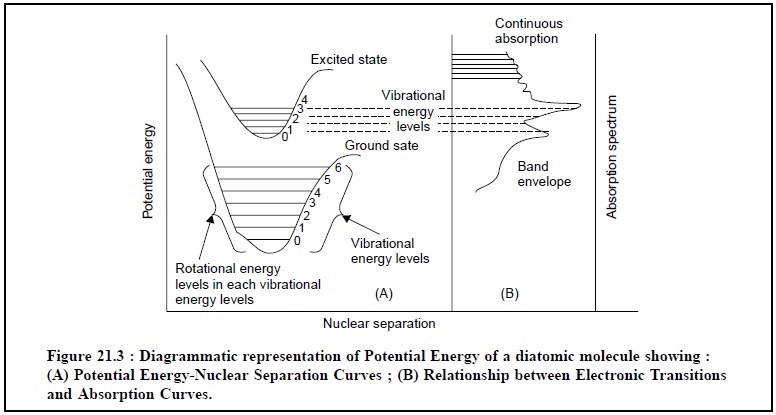
Explanation of various features in Figures 21.3 :
(i) The mutual
forces are also zero when the nuclei are at infinity ; but as the latter come
closer to one another, forces of attraction start operating and the potential
energy decreases,
(ii) The
potential energy records an increase when the nuclei get very close to one
another thereby causing repulsion,
(iii) The
atoms, therefore, can vibrate about the minimum position RC at the vibrational
level 0,
(iv) The
electronic configuration of the molecule gives rise to different quantum of
energy associated with it which may be indicated and represented by the
horizontal lines in Figure 21.3 (0 → 6),
(v) At ambient
temperature, the molecule is in the lowest ebb of the vibrational level of the
ground state,
(vi) The
corresponding electronic transition from the ground state to an excited state,
is represented by the upper curve in Figure 21.3,
(vii)
Rotational energy variations usually accompany electronic variations, however,
they are compara-tively smaller in size and often yield a fine structure
superimposed on the electronic-vibrational change,
(viii) The
frequency of the absorption bands associated with the transition is put forward
by the follow-ing expression :
hν =
EExcited state – EGround
state ...(e)
where, h = Planck’s Constant,
v= Frequency, and E = Energy
level.
In reality, their appearance as a pattern comes into
being chiefly from transitions to the various vibrational levels of the excited
state as shown in Figure 21.3.
8. FACTORS INFLUENCING ABSORPTION OF RADIANT ENERGY
There are various cardinal factors that govern
measurement of absorption of radiant energy, namely :
(a) Absorbing
groups (or Chromophores),
(b) Solvent
effects,
(c) Effect of
temperature, and
(d) Inorganic
ions.
These vital factors would be discussed briefly with
specific examples hereunder :
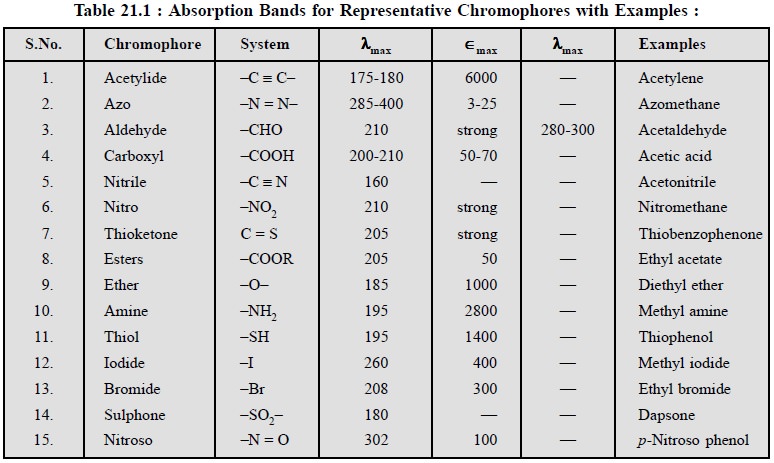
8.1. Absorbing Groups (or Chromophores)
A ‘chromophore’
is a group which when attached to a saturated hydrocarbon produces a molecule
that absorbs a maximum of visible of UV energy at some specific wavelength.
A few typical examples having electronic absorption bands
for various representive chromophores are provided in the following Table : 21
: 1 :
8.2. Solvent Effects
The absorption spectrum of a pharmaceutical substance
depends partially upon the solvent that has been employed to solubilize the
substance. A drug may absorb a miximum of radiant energy at a particular
wavelength in one solvent but shall absorb practically little at the same
wavelength in another solvent. These apparent changes in spectrum are
exclusively due to various characteristic features, namely :
(a) Nature of
the solvent,
(b) Nature of
the absorption band, and
(c) Nature of
the solute.
Some salient features of ‘Solvent Effects’ are enumerated below :
(i)
Absorption bands of many substances are relatively
sharper and may also exhibit fine structure when measured in solvents of low
dipole moment,
(ii) Interactions of
solvent-solute are found to be much stronger in such substances where strong
dipole forces are involved,
(ii)
Solvent effects do help
in reorganizing electronic transitions of the type n—π* that essentially
involve the nonbonding electrons of nitrogen and oxygen,
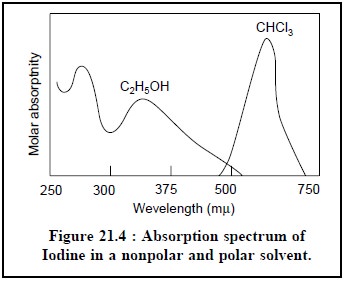
(iv) The nonbonding
electrons of nitrogen and oxygen usually interact with polar solvents that
ultimately give rise to a characteristic shift to shorter wavelengths.Example :
The spectrum of Iodine in a nonpolar solvent like CHCl3 is found to be
distinctly different (purple to the naked eye) when the same is compared in a
polar solvent such as C2H5OH (brownish to the naked eye) in Figure 21.4.
(v) A spectrum
normally shows appreciable changes with varying pH when an
ionizable moiety is present in the molecule and thereby constitutes part of the
chromophore structure.
8.3 Effect of Temperature
·
Low temperature offfer sharper absorption bands of many
pharmaceutical substances than at room temperature,
·
Vibrational resolutions are definitely well-defined at
low temperatures because of the following two reasons, namely :
(a) Fewer vibrational levels are occupied, and
(b) Degree of solute-solvent interaction is minimised,
·
Samples in highly rigid or viscous media (e.g., glass) is examined frequently in
phosphorescence methods and also in some fluorescence methods.
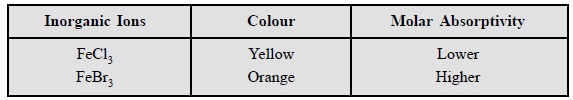
8.4 Inorganic Ions
The ‘chromophoric entities’ present in the inorganic
compounds are of two types, namely :
(a) Involving several atoms : such as :
permanganate (MnO4–) and dichromate (Cr2O7–)
moieties, and
(b) Involving single atoms : Those having
incomplete outer d-electron shells
where closely spaced, unoccupied energy levels are available in abundance for
instance : coordination compounds with
Rare Earths : e.g.,
Be, Sr, Ra, and Transition Elements : Cr, Mn, Ni, Pt, Ag, Pd, Cd, Hg, Au,
It is worth while to note that the absorption spectra for
these elements are caused due to a charge-transfer-process whereby an electron gets
transferred form one part of the ion to another.
Interestingly, inclusion of readily polarizable atoms do
exert an effect likewise to lengthening a con-jugated chain. Examples :
Related Topics