Chapter: Physics : Electromagnetic Waves and Wave optics : Higher Secondary(12 Std)
Types of spectra
Types of spectra
When white light falls on a prism, placed in a spectrometer, the waves of different wavelengths are deviated to different directions by the prism. The image obtained in the field of view of the telescope consists of a number of coloured images of the slit. Such an image is called a spectrum.
If the slit is illuminated with light from sodium vapour lamp, two images of the slit are obtained in the yellow region of the spectrum. These images are the emission lines of sodium having wave lengths 5896Ao and 5890Ao. This is known as spectrum of sodium.
The spectra obtained from different bodies can be classified into two types (i) emission spectra and (ii) absorption spectra.
(i) Emission spectra
When the light emitted directly from a source is examined with a spectrometer, the emission spectrum is obtained. Every source has its own characteristic emission spectrum.
The emission spectrum is of three types.
1. Continuous spectrum
2. Line spectrum and
3. Band spectrum
1. Continuous spectrum
It consists of unbroken luminous bands of all wavelengths containing all the colours from violet to red. These spectra depend only on the temperature of the source and is independent of the characteristic of the source.
Incandescent solids, liquids, Carbon arc, electric filament lamps etc, give continuous spectra.
Line spectrum
Line spectra are sharp lines of definite wavelengths. It is the characteristic of the emitting substance. It is used to identify the gas.

Atoms in the gaseous state, i.e. free excited atoms emit line spectrum. The substance in atomic state such as sodium in sodium vapour lamp, mercury in mercury vapour lamp and gases in discharge tube give line spectra (Fig. 5.4).
Band Spectrum
It consists of a number of bright bands with a sharp edge at one end but fading out at the other end.
Band spectra are obtained from molecules. It is the characteristic of the molecule. Calcium or Barium salts in a bunsen flame and gases like carbon−di−oxide, ammonia and nitrogen in molecular state in the discharge tube give band spectra. When the bands are examined with high resolving power spectrometer, each band is found to be made of a large number of fine lines, very close to each other at the sharp edge but spaced out at the other end. Using band spectra the molecular structure of the substance can be studied.
(ii) Absorption Spectra
When the light emitted from a source is made to pass through an absorbing material and then examined with a spectrometer, the obtained spectrum is called absorption spectrum. It is the characteristic of the absorbing substance.
Absorption spectra is also of three types
1. continuous absorption spectrum
2. line absorption spectrum and
3. band absorption spectrum
Continuous absorption spectrum
A pure green glass plate when placed in the path of white light, absorbs everything except green and gives continuous absorption spectrum.
Line absorption spectrum
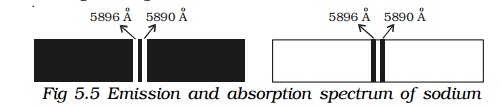
When light from the carbon arc is made to pass through sodium vapour and then examined by a spectrometer, a continuous spectrum of carbon arc with two dark lines in the yellow region is obtained as shown in Fig.5.5.
Band absorption spectrum
If white light is allowed to pass through iodine vapour or dilute solution of blood or chlorophyll or through certain solutions of organic and inorganic compounds, dark bands on continuous bright background are obtained. The band absorption spectra are used for making dyes.
1 Fraunhofer lines
If the solar spectrum is closely examined, it is found that it consists of large number of dark lines. These dark lines in the solar spectrum are called Fraunhofer lines. Solar spectrum is an example of line absorption spectrum.
The central core of the sun is called photosphere which is at a very high temperature of the order of 14 million kelvin. It emits continuous spectrum. The sun’s outer layer is called chromosphere. This is at a comparatively lower temperature at about 6000 K. It contains various elements in gaseous state.
When light from the central core of the sun passes through sun’s atmosphere, certain wavelengths are absorbed by the elements present in the chromosphere and the spectrum is marked by dark lines.
By comparing the absorption spectra of various substances with the Fraunhofer lines in the solar spectrum, the elements present in the sun’s atmosphere have been identified.
2. Fluorescence
When an atomic or molecular system is excited into higher energy state by absorption of energy, it returns back to lower energy state in a time less than 10−5 second and the system is found to glow brightly by emitting radiation of longer wavelength.
When ultra violet light is incident on certain substances, they emit visible light.
It may be noted that fluorescence exists as long as the fluorescing substance remain exposed to incident ultraviolet light and re-emission of light stops as soon as incident light is cut off.
3. Phosphorescence
There are some substances in which the molecules are excited by the absorption of incident ultraviolet light, and they do not return immediately to their original state. The emission of light continues even after the exciting radiation is removed. This type of delayed fluorescence is called phosphorescence.
Related Topics