Chapter: Physics : Electromagnetic Waves and Wave optics : Higher Secondary(12 Std)
Scattering of light
Scattering of light
Lord Rayleigh was the first to deal with scattering of light by air molecules. The scattering of sunlight by the molecules of the gases in Earth’s atmosphere is called Rayleigh scattering.
The basic process in scattering is absorption of light by the molecules followed by its re-radiation in different directions. The strength of scattering depends on the wavelength of the light and also the size of the particle which cause scattering.
The amount of scattering is inversely proportional to the fourth power of the wavelength. This is known as Rayleigh scattering law. Hence, the shorter wavelengths are scattered much more than the longer wavelengths. The blue appearance of sky is due to scattering of sunlight by the atmosphere. According to Rayleigh’s scattering law, blue light is scattered to a greater extent than red light. This scattered radiation causes the sky to appear blue.
At sunrise and sunset the rays from the sun have to travel a larger part of the atmosphere than at noon. Therefore most of the blue light is scattered away and only the red light which is least scattered reaches the observer. Hence, sun appears reddish at sunrise and sunset.
1. Tyndal scattering
When light passes through a colloidal solution its path is visible inside the solution. This is because, the light is scattered by the particles of solution. The scattering of light by the colloidal particles is called Tyndal scattering.
2. Raman effect
In 1928, Sir C.V. Raman discovered experimentally, that the monochromatic light is scattered when it is allowed to pass through a substance. The scattered light contains some additional frequencies other than that of incident frequency. This is known as Raman effect.
The lines whose frequencies have been modified in Raman effect are called Raman lines. The lines having frequencies lower than the incident frequency are called Stoke’s lines and the lines having frequencies higher than the incident frequency are called Anti−stokes lines. This series of lines in the scattering of light by the atoms and molecules is known as Raman Spectrum.
The Raman effect can be easily understood, by considering the scattering of photon of the incident light with the atoms or molecules. Let the incident light consist of photons of energy hνo.
1. If a photon strikes an atom or a molecule in a liquid, part of the energy of the incident photon may be used to excite the atom of the liquid and the rest is scattered. The spectral line will have lower frequency and it is called stokes line.
2. 1. If a photon strikes an atom or a molecule in a liquid, which is in an excited state, the scattered photon gains energy. The spectral line will have higher frequency and it is called Anti−stoke’s line.
3. In some cases, when a light photon strikes atoms or molecules, photons may be scattered elastically. Then the photons neither gain nor lose energy. The spectral line will have unmodified frequency.
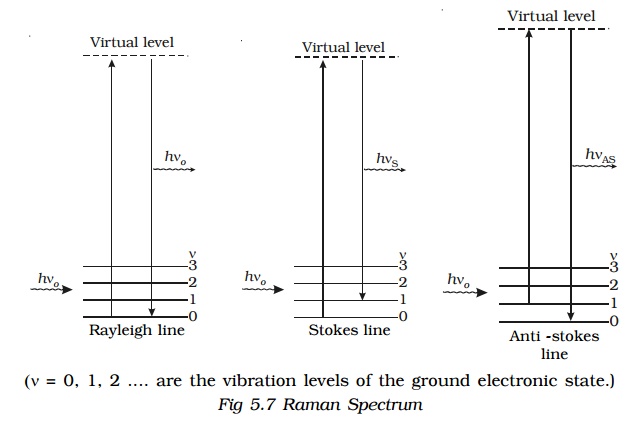
If νo is the frequency of incident radiation and νs the frequency of scattered radiation of a given molecular sample, then Raman Shift or Raman frequency ∆ν is given by the relation ∆ν = νο − νs.
The Raman shift does not depend upon the frequency of the incident light but it is the characteristic of the substance producing Raman effect. For Stoke’s lines, ∆ν is positive and for Anti–stoke’s lines ∆ν is negative.
The intensity of Stoke’s line is always greater than the corresponding Anti−stoke’s Line. The different processes giving rise to Rayleigh, Stoke’s and Anti-stokes lines are shown in Fig 5.7.
When a system interacts with a radiation of frequency νo, it may make an upward transition to a virtual state. A virtual state is not one of the stationary states of the molecule. Most of the molecules of the system return back to the original state from the virtual state which corresponds to Rayleigh scattering. A small fraction may return to states of higher and lower energy giving rise to Stoke’s line and Anti-stoke’s line respectively.
3. Applications of Raman Spectrum
1. It is widely used in almost all branches of science.
2. Raman Spectra of different substances enable to classify them according to their molecular structure.
3. In industry, Raman Spectroscopy is being applied to study the properties of materials.
4. It is used to analyse the chemical constitution.
Related Topics