Chapter: Modern Pharmacology with Clinical Applications: Drugs Used in Mood Disorders
Treatment of Manic-Depressive Illness
TREATMENT OF
MANIC-DEPRESSIVE ILLNESS
Lithium
For more than 40 years, Li+
has been used to treat ma-nia. While it is relatively inert in individuals
without a mood disorder, lithium carbonate is effective in 60 to 80% of all
acute manic episodes within 5 to 21 days of beginning treatment. Because of its
delayed onset of ac-tion in the manic patient, Li+ is often used in
conjunc-tion with low doses of high-potency anxiolytics (e.g., lo-razepam) and
antipsychotics (e.g. haloperidol) to stabilize the behavior of the patient.
Over time, in-creased therapeutic responses to Li+ allow for a
gradual reduction in the amount of anxiolytic or neuroleptic re-quired, so that
eventually Li+ is the sole agent used to maintain control of the
mood disturbance.
In addition to its acute
actions, Li+ can reduce the frequency of manic or depressive
episodes in the bipolar patient and therefore is considered a mood-stabilizing
agent. Accordingly, patients with bipolar disorder are often maintained on low
stabilizing doses of Li+ indefi-nitely as a prophylaxis to future
mood disturbances. Antidepressant medications are required in addition to Li+
for the treatment of breakthrough depression.
Mechanism of Action
Lithium is a monovalent
cation that can replace NA+ in some biological processes. It can be
argued that compe-tition by Li+ for active NA+ sites may
lead to altered neuronal functions that may account for its antimanic and
mood-stabilizing actions. In this regard, the failure of Li+ to
maintain a normal membrane potential be-cause of its lower affinity for the NA+
pump has been demonstrated. However, this action of Li+ would not
explain its relatively selective effects on the CNS, spar-ing comparable
excitable tissues (e.g. cardiac muscle) in the periphery. Moreover, an action
on membrane polar-ity would be so general that the entire pool of brain neurons
would be affected by Li+ . It seems more rea-sonable that Li+
produces its psychotropic actions by perturbation of molecular events common to
a few CNS synapses that might have been disturbed during the course of the
manic-depressive illness.
Recently, attention has
focused on the actions of Li+ on receptor-mediated second-messenger
signaling sys-tems of the brain. In this regard, interactions between Li+
and guanine nucleotide (GTP) binding proteins (G proteins) have been the target
of many studies, since G proteins play a pivotal role in the function of many
sec-ond-messenger signaling systems. Lithium is capable of altering G-protein
function. It can diminish the cou-pling between the receptor recognition site
and the G protein. The molecular mechanism involves the compe-tition for Mg
sites on the G protein, which are essen-tial for GTP binding. Guanine
nucleotide activates the G protein. Accordingly, in the presence of Li+
, receptor-mediated activation of these G proteins is attenuated. This action
of Li+ has been selectively demonstrated for G proteins associated
with β-adrenoceptors and M1
muscarinic receptors of the CNS (Fig. 33.4).
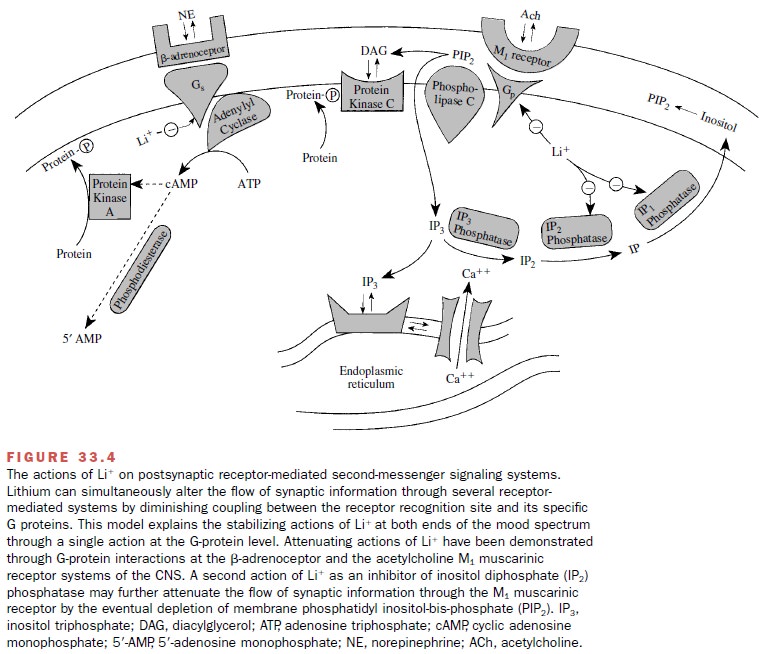
While it is not possible at
present to assign a thera-peutic role to this action of Li+ , it is
a step toward ex-plaining the stabilizing actions of this drug. Since sev-eral
neurotransmitter receptors share common G protein–regulated second-messenger
signaling systems, Li+ could simultaneously correct the alterations
at indi-vidual synapses associated with depression and mania by a single action
on the function of specific G proteins.
An additional action of Li+
is interruption of the phosphatidylinositide cycle through an inhibitory action
on inositol phosphate metabolism. By this mechanism, depletion of membrane
inositol and the phosphoinosi-tide-derived second-messenger products
diacylglycerol and inositol triphosphate ultimately reduces signaling through
receptor systems dependent on the formation of these products. It is presently
unclear to what extent inhibition of inositol phosphate metabolism contributes
to the therapeutic properties of Li+ in bipolar patients.
Pharmacokinetics and Therapeutic Drug Monitoring
Lithium is readily absorbed
from the gastrointestinal tract, reaching a peak plasma level in 2 to 4 hours.
Distribution occurs throughout the extracellular fluid with no evidence of
protein binding. Passage through the blood-brain barrier is limited, so that
cerebrospinal fluid levels are 50% of plasma levels at steady state.
The elimination half-life of Li+ is estimated at 24 hours, and more than 90% of the dose of Li+ is excreted into the urine. Renal clearance, however, is only 20%, since Li+ is actively reabsorbed in the proximal tubule at sites normally used for the conservation of NA+ . Thus, competition between Li+ and NA+ for uptake sites can alter the elimination of Li+ and its concentration in to-tal body water. NA+ loading enhances Li+ clearance, while NA+ depletion promotes Li+ retention. This im-portant relationship explains the appearance of Li+ tox-icity (discussed later) associated with diet (low NA+ ), drugs (diuretics), medical conditions (diarrhea), or physical activities (those that induce sweating) that de-plete the body of NA+ .
The elimination rate of Li+
from the body is vari-able. It is quite rapid during the first 10 hours after
in-gestion, and this period accounts for about 40% of the total Li+ excretion.
However, the remaining portion of the Li+ dose is excreted very
slowly over 14 days. Because of this biphasic elimination rate, clinically
use-ful serum Li+ concentrations are usually determined 12 hours
after the last dose. This period assures a relatively accurate reflection of
the Li+ concentration, since it is beyond the most variable portion
(rapid elimination phase) of the Li+ elimination profile.
Adverse Effects
The frequency and severity of
adverse reactions associ-ated with Li+ therapy are directly related
to serum lev- els. Since Li+ has a low therapeutic index
(approxi-mately 3) and a narrow therapeutic window (0.5–1.5 mEq/L), the
frequent measurement of serum steady-state concentrations is standard practice
in the treat-ment of bipolar patients.
Adverse reactions occurring
at serum trough levels (12 hours after the last dose) below 1.5 mEq/L are
gen-erally mild, whereas those seen above 2.5 mEq/L are usually quite severe.
Mild toxicity is usually expressed as nausea, vomiting, abdominal pain,
diarrhea, polyuria, sedation, and fine tremor. If the serum concentration of Li+
progressively rises above 2 mEq/L, frank neurolog-ical toxicity appears,
beginning with mental confusion and progressing to hyperreflexia, gross tremor,
dysarthria, focal neurological signs, seizures, progressive coma, and even
death.
Adverse effects sometimes
seen during chronic maintenance of bipolar patients with Li+ include
hy-pothyroidism (approximately 5%) and nephrogenic dia- betes insipidus. Both
conditions are readily reversible by discontinuation of Li+ .
Routine laboratory monitoring includes TSH (thyroid-stimulating hormone) and
serum creatinine measurements to detect hypothyroidism and any change in renal
capacity to clear the drug.
Other Mood-Stabilizing Agents
Several anticonvulsant
medications have mood-stabi-lizing properties. Valproic acid and carbamazepine
are the best studied to date. In 1995, valproic acid was ap-proved by the FDA
for treatment of acute mania and is now considered a first-line agent. Other
anticonvul-sants under investigation include lamotrigine and top-iramate.
Related Topics