Chapter: Modern Pharmacology with Clinical Applications: Drugs Used in Mood Disorders
Treatment of Major Depression: The Selective Serotonin Reuptake Inhibitors
The Selective Serotonin Reuptake
Inhibitors
In 1987, the FDA approved the
drug fluoxetine (Prozac) for use in
the treatment of major depression. Fluoxetine belongs to a class of agents
referred to as selective sero-tonin
reuptake inhibitors (SSRIs). The SSRIs now in-clude sertraline (Zoloft), fluvoxamine (Luvox), paroxe-tine (Paxil), and citalopram (Celexa).
With the introduction of the
SSRIs, the safety and tolerability of antidepressants improved remarkably. As a
class, these medications have little or no affinity for cholinergic, β-adrenergic or histamine
receptors and do not interfere with cardiac conduction. They are well
tol-erated by patients with heart disease and by the elderly, who are
especially sensitive to the anticholinergic and orthostatic effects of the
tricyclic antidepressant agents (TCAs) and monoamine oxidase inhibitors
(MAOIs).
The high degree of
selectivity of SSRIs for the nerve terminal serotonin reuptake system has
supported the hypothesis that these agents produce their therapeutic action
through an ability to modulate serotonin neuro-transmission in the brain.
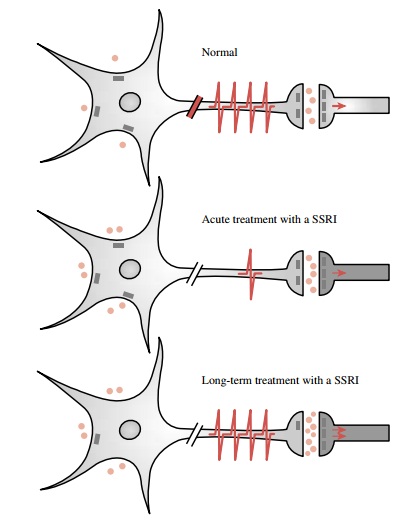
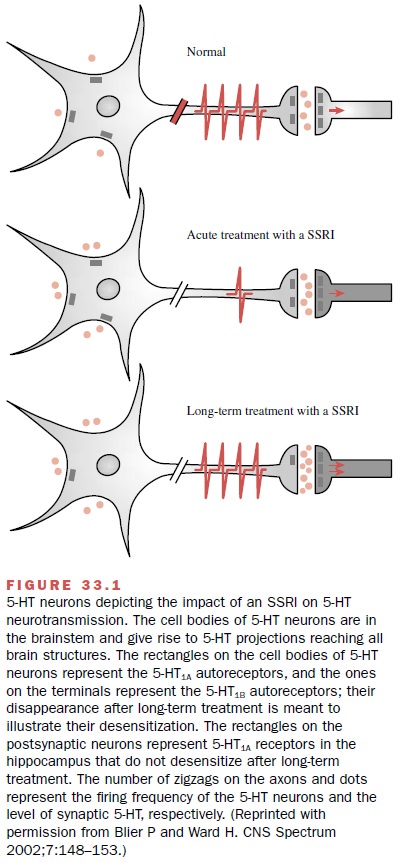
Chronic studies in animals have provided evidence for a cascade of altered
synap-tic events, beginning with inhibition of 5-hydroxytrypta-mine (5-HT)
neuronal reuptake (Fig. 33.1). Increased 5-HT levels activate 5-HT1A
autoreceptors and result in a decrease in neuronal firing. Desensitization of
this re-ceptor results in enhanced serotonin release. The termi-nal 5-HT1B
autoreceptors normally inhibiting release of serotonin also become
desensitized. These events, trig-gered by a sustained inhibition of the nerve
terminal serotonin reuptake system, ultimately cause a potentia-tion of
serotonin neurotransmission at central synaptic sites.
The development of
these synaptic events shares the time frame of the delayed appearance of the
thera-peutic benefit of these agents in depression.
With initiation of therapy
with an SSRI, some pa-tients describe anxiety or agitation. This can usually be
overcome by reducing the dose and titrating upward more slowly. Insomnia can be
a persistent activating side effect that can limit therapy or require the
addition of a sedating agent at bedtime. Nausea and loose stools are a frequent
side effect and may be lessened by taking the medication with food. As many as
one-third of pa-tients taking SSRIs may complain of sexual dysfunc-tion,
including decreased libido, delayed ejaculation, and anorgasmia. The SSRIs tend
to be weight neutral with the exception of paroxetine (Paxil), which is asso-ciated with weight gain. No correlation has
been made between plasma levels of the SSRIs and efficacy.
Fluoxetine
Fluoxetine (Prozac) is given in the morning because
of its potential for being activating and causing insomnia. Food does not
affect its systemic bioavailability and may actually lessen the nausea reported
by some pa-tients. Fluoxetine is highly bound to serum proteins and may
interact with other highly protein bound drugs. It is demethylated in the liver
to form an active metabolite, norfluoxetine. Inactive metabolites are excreted
by the kidney. Doses must be reduced in patients with liver dis-ease.
The slow elimination of
fluoxetine and norfluoxe-tine lead to special clinical concerns when adjusting
doses and discontinuing this medication. Steady state is not reached until 4 to
6 weeks, and similarly, complete elimination takes 4 to 6 weeks after
discontinuation of the medication. A 4- to 6-week waiting period should be
permitted before starting a medication with potential for an interaction with
fluoxetine, such as a monoamine oxidase inhibitor (MAOI). Additionally,
fluoxetine is a potent inhibitor of cytochrome P450 2D6 and can sig-nificantly
elevate levels of drugs metabolized by this route. Thus, coadministration of
drugs with a narrow therapeutic index, such as TCAs and type 1C
antiar-rhythmics, including flecainide and propafenone, are a particular
concern.
Sertraline
Sertraline (Zoloft) has an elimination half-life of
25 hours and can be administered once a day, usually in the morning to avoid
insomnia. Sertraline undergoes exten-sive hepatic metabolism, and doses must be
reduced in patients with liver disease. Sertraline may produce more
gastrointestinal side effects, such as nausea and diar-rhea, than does
fluoxetine and is generally thought to be less activating than fluoxetine. It
is highly bound to serum proteins (98%) and may alter plasma protein binding of
other medications. A 14-day washout period is recommended before starting a
MAOI. Sertraline is a weak inhibitor of cytochrome P450 2D6. Intensive
ther-apeutic drug monitoring is indicated when combining sertraline with drugs
metabolized by this route that have a narrow therapeutic index, such as the
TCAs and the type 1C antiarrhythmics propafenone, encainide, and flecainide.
Paroxetine
Paroxetine (Paxil) has an elimination half-life of
21 hours and is also highly bound to plasma proteins, so it requires special
attention when administered with drugs such as warfarin. Paroxetine is a potent
inhibitor of the cytochrome P450 2D6 isoenzyme and can raise the plasma levels
of drugs metabolized via this route. Of particular concern are drugs with a
narrow therapeutic index, such as TCAs and the type 1C antiarrhythmics
flecainide, propafenone, and encainide. Additionally, paroxetine itself is
metabolized by this enzyme and in-hibits its own metabolism, leading to
nonlinear kinetics. Weight gain is higher with paroxetine than with the other
SSRIs, and it tends to be more sedating, presum-ably because of its potential
anticholinergic effects. Additionally, patients have had difficulty with abrupt
discontinuation with this agent, reporting a flulike syn-drome; this symptom
can be avoided by tapering the medication.
Citalopram
Citalopram (Celexa) has an elimination half-life of
35 hours and is 80% bound to plasma proteins. Of all of the SSRIs it has the
least effect on the cytochrome P450 sys-tem and has the most favorable profile
regarding drug–drug interactions.
Related Topics