Chapter: Pharmaceutical Drug Analysis: Thermoanalytical Analysis
Thermometric Titrations (TT)
THERMOMETRIC TITRATIONS (T T)
1. THEORY
The thermometric titrations (TT) make use of ‘heats of reaction’ to obtain titration
curves. In usual practice, the temperature of solution is plotted against the
volume of titrant. TT is performed by allowing the titrant to flow from a
thermostated-burette directly into a solution contained in a
thermally-insulated vessel, and subsequently the observed change in temperature
of the solution is recorded precisely either during con-tinuous addition of
titrant or after every successive incremental addition. The end-point is aptly
indicated by a sharp break in the curve.
As the dielectric constant of a solvent exerts little
effect on the thermometric titrations, the latter may be employed effectively
in most non-aqueous media.
Hence, in a broader-sense TT may be utilized in a number
of reactions with greater efficacy, for in-stance : complexation,
precipitation, redox, neutralization. Further, TT can be used to titrate gases
against other gases devoid of a liquid-phase ; and to titrate liquid solutions
with gaseous reagents.
2. INSTRUMENTATION
A standard thermometric titration assembly essentially
consists of the following four vital
components, namely :
(i)
Motor-driven Burette,
(ii) Adiabatic
Titration Chamber
(iii)
Thermister Bridge Assembly, and
(iv) Recorder.
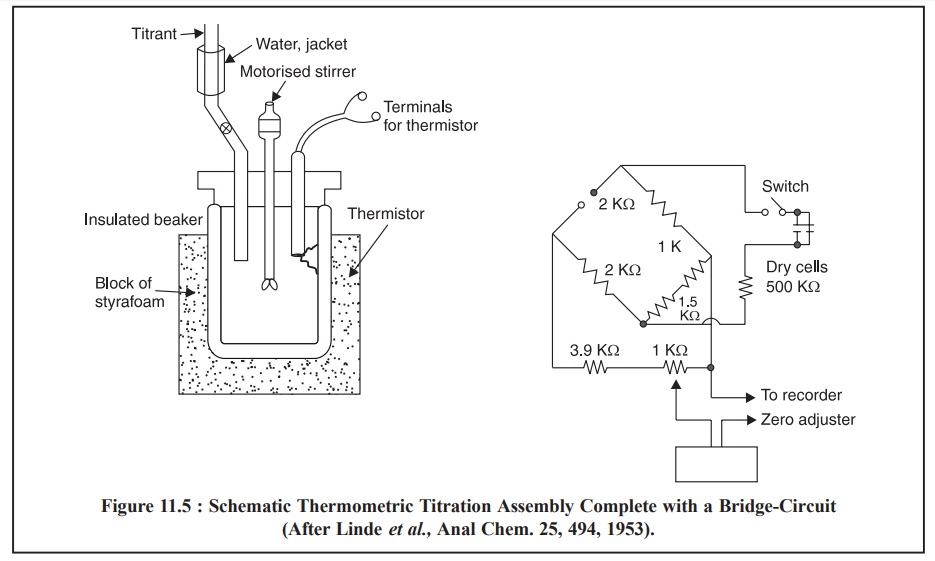
Fiaure 11.5, represents the schematic thermometric titration
assembly complete with a bridge-circuit. To minimise heat transfer losses from
the solution by its immediate surroundings, the thermometric titrations are
usually carried out in an isolated-beaker tightly closed with a stopper having
provision for a burette-tip, a motorized-glass stirrer, and a
temperature-monitoring arrangement.
Procedure :
(a) Introduce
the titrant from a burette that is duly mounted in a thermostated-water-jacket
to maintain the temperature of the titrant within ± 0.05°C,
(b)
Experimental parameters are predetermined in such a fashion such that the
volume of titrant needed for each titration must lie between 1-3 ml,
(c) Automated
device delivering reagent at a steady and constant rate of 600 µl
per minute usually permits recording,
(d)
Constant-speed motorized stirrer at 600 rpm is employed to effect uniform
mixing of solution,
(e) Variations
in temperature are measured with the help of a sensitive
thermister-sensing-element with fast response, that is sealed completely in
glass and immersed in solution,
(f) In the
course of a thermometric titration, the thermister attached to the
insulated-beaker is connected to one arm of the Wheatstone Bridge as displayed
in Figure 11.5. The values of the circuit component listed are for a thermister
having an approximate resistance of 2 KΩ
and a sensitivity of –0.04 Ω/Ω/°C
in the 25°C temperature range. Hence, an observed change of 1°C an unbalanced
potential of 15.7 mV, and
(g) The heat of reaction is
either absorbed or generated upon addition of the titrant to the beaker,
thereby unbalancing the Wheatstone Bridge caused by simultaneous variations in
the resistance (temperature) in the insulated-beaker thermister. Thus, the
bridge unbalance potential is promptly plotted by the recorder.
Note : (i) To minimise the
temperature variations between the titrant and the solution and also to obviate
volume corrections, the concentration of the titrant is invariably maintained
10–100 times higher than that of the reactant, and
(ii) To obtain optimum results, temperatures of
the titrant and the solution must be always within 0.2°C of each other before a titration is commenced.
3. METHODOLOGY
Thermometric titration curves usually represent both the
entropy and the free energy involved. The titrant is added to the solution at a
constant rate in order that the voltage output of the
thermister-temperature-transducer changes linearly with time upto the
equivalence point.
TT-method affords exact end-point due to :
(a) Coloured
solutions, and
(b) Poisoning
of Electrodes.
In usual practice it has been observed that thermometric
titrations are mostly feasible with such sys-tems that provide rates of
temperature change more than 0.01°C/second.
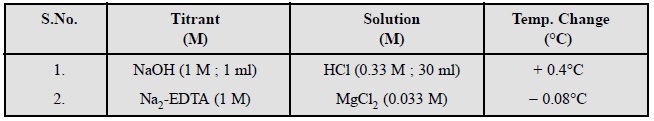
A few typical examples are cited below :
Precautions :
(i) Lower limit
of concentrations which can be titrated effectively is 0.002 M,
(ii) No
transfer of heat between the titration vessel and its immediate surroundings is
allowed, and
(iii) During
titration temperature fluctuation must not exceed 0.001°C.
4. APPLICATIONS
Various important applications of thermometric titrations
are enumerated below :
(i) Precipitation Reactions : e.g., Chloride ions (Cl–)
with Ag+ ions. Besides, phase relations have been studied extensively
in precipitation reactions.
(ii) Ion-combination Reactions : e.g., divalent cations like Ca2+,
Mg2+ with EDTA (complexometric estimation),
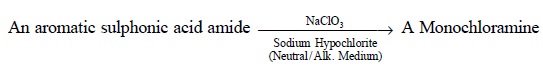
(i)
Conversion of Amides to Amines
: e.g.,
(iv) Estimation of H2O and (CH3CO)2O
concentrations in a mixture : The concentration of either of these
reactions in the presence of the other may be determined successfully by
measuring the rise in temperature taking place during the exothermic reactions
of water and acetic anhydride in gla-cial acid solution along with a trace of
perchloric acid (HClO4) acting as a catalyst, and
(v) Benzene in Cyclohexane : Benzene may be
estimated rapidly with fairly good accuracy in cyclohexane by measuring the
heat of nitration, whereby a previously prepared standard nitrating acid
mixture (benzene and cyclohexane) and the subsequent temperature rise is noted
which is a direct function of the quantity of benzene present.
Details involving various experimental parameters for the
above estimation are enumerated below :
4.1. Estimation of Benzene in Cyclohexane
Materials Required : Thermometric titration
assembly as per Figure 11.5, minus the burette; a stop-watch or timer ;
standard nitrating acid mixture [mix 2 volumes of 70% HNO3 (d = 1.41) with 1 volume of 95% H2SO4
(d = 1.82)] ; Bakelite screw-cap
bottle (4 oz. capacity) : 2.
Procedure :
·
Weigh 50 g of sample in a Bakelite screw-cap bottle and
in a similar bottle put the standard nitrating mixture. Place these two bottles
in a thermostat maintained at 20°C until the contents have attained an
equilibrium temperature,
·
Transfer 50 ml of the standard nitrating-acid to the
insulated vessel and insert the motorised stirrer. Just wait for about 3-5
minutes and then start the motorized stirrer. After exactly 1 minute record the
initial temperature,
·
Stop the motor. Insert the sample into the reaction
vessel and start the stirrer. Now, start taking readings of the rise in
temperature after each interval 1, 2, 3 and 5 minutes respectively, and
·
Plot a ‘calibration curve’ between the observed
temperature-rise in a 3 minute interval Vs
percent benzene present in cyclohexane. Run pure cyclohexane and standards
containing 0.5-5.0 percent benzene by weight.
Related Topics