Chapter: Pharmaceutical Drug Analysis: Thermoanalytical Analysis
Thermogravimetric Analysis (TGA)
THERMOGRAVIMETRIC ANALYSIS (TGA)
1. THEORY
A large number of chemical substances invariably
decompose upon heating, and this idea of heating a sample to observe weight
changes is the underlying principle of thermogravimetric analysis (TGA).
However, TGA may be sub-divided into two
heads, namely :
(a) Static (or
Isothermal) Thermogravimetric Analysis, and
(b) Dynamic
Thermogravimetric Analysis.
1.1. Static Thermogravimetric Analysis
In this particular instance the sample under analysis is
maintained at a constant temperature for a period of time during which any
changes in weight are observed carefully.
1.2. Dynamic Thermogravimetric Analysis
In dynamic thermogravimetric analysis a sample is
subjected to conditions of predetermined, carefully controlled continuous
increase in temperature that is invariably found to be linear with time.
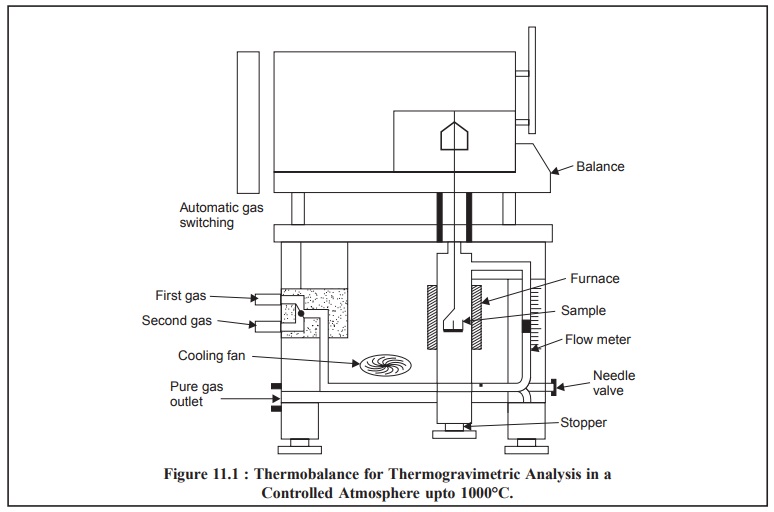
2. INSTRUMENTATION
The essential requirements for an instrument (Figure
11.1) meant for thermogravimetric analysis are, namely :
(a) A
high-precision balance,
(b) A furnace
adequately programmed for a linear rise of temperature with time, and
(a)
A sensitive recorder.
2.1. Balances
They are usually of two
types :
(a) Null-point Type : It makes use of an
appropriate sensing-element which aptly detects any slightest deviation of the
balance beam and provides the application of a restoring force, directly
propor-tional to the change in weight, thereby returning the beam to its
original null-point. The restoring-force is subsequently recorded either
directly or with the aid of a transducer.
(b) Deflection Type : It is essentially
based on either a conventional analytical balance consisting of helical spring,
cantilever beam and strain gauze or a torsion analytical balance involving the
conversion of deviations directly into a record of the weight change.
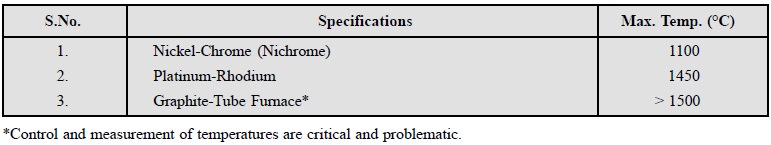
2.2. Furnace
The furnace must be designed in such a fashion so as to
incorporate an appropriate smooth input thereby maintaining either a fixed
temperature or a predetermined linear-heating programme (e.g., 10°C-600°C per hour).
The temperature control of the furnace is satisfactorily
achieved via a thermocouple mounted very close to the furnace-winding. The
maximum operational temperature may be obtained by using different
thermocouples as indicated below :
2.3. Recorder
The recording device must be such so as to :
(i) record both
temperature and weight continuously, and
(ii) make a
definite periodic record of the time.
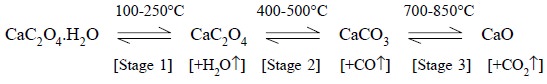
3. METHODOLOGY
The ‘thermogram’
for calcium oxalate monohydrate (CaC2O4.H2O)
is presented in Figure 11.2. The successive plateaus correspond to the
anhydrous oxalate (100-250°C), calcium carbonate (400-500°C), and finally
calcium oxide (700-850°C). In other words, these plateaus on the decomposition
curve designate two vital aspects,
namely :
(a) clear
indication of constant weight, and
(b) stable
phases within a specified temperature interval. The chemical reactions involved
may be summarized as follows :
3.1. Interpretation of Thermogram
In the thermogram
(Figure 11.2), which
vividly illustrates the
thermogravimetric evaluation of CaC2O4.H2O,
it is ensured that the weight of this product decreases in several stages,
namely :
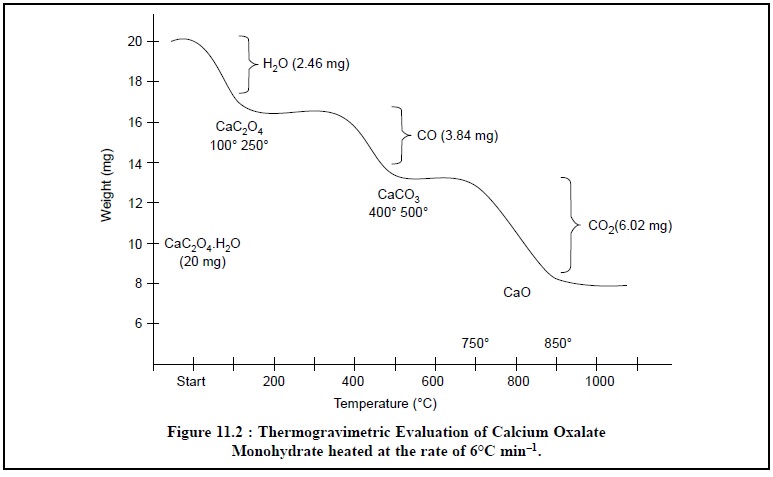
Stage 1 : The water of hydration (or
crystallization) from calcium oxalate monohydrate is lost which corresponds to 2.46 mg (12.3%) equivalent
to 1 mole of H2O in the temperature range 100-250°C.
Actually, the 12.3% weight loss that took place between
100-250°C should correspond to 12.3% of the original formula weight for CaCO3
H2O (FW = 146). Hence, the product being lost has a formula weight
of 0.123 × 146 = 17.958 ( ~ 18.0), and it corresponds to H2O.
Stage 2 : One mole of carbon monoxide is
evolved subsequently from calcium oxalate, corresponding to 3.84 mg (19.2%) in the temperature range 400-500°C.
The 19.2% weight loss that occurred between 400-500°C
should correspond to 19.2% of the original formula weight of 146. Therefore,
the product being given out has a formula weight of 1.92 × 146 = 28.0, that
corresponds to CO.
Stage 3 : Finally, a mole of CO2 is evolved from calcium carbonate that
corresponds to 6.02 mg (3.01%) in
the temperature range 700-850°C.
The weight loss amounting to 3.01% which took place in
the range 700-850°C must, in fact, corresponds to 3.01% of the original formula
weight of 146. Therefore, the product being evolved has a formula weight of
0.301 × 146 = 43.946 ( ~ 44), and it corresponds to CO2.
![]()
It is quite evident that in a multicomponent system
wherein more than one component exhibits weight variations and that too at
different temperature regions, the composition of the original compound may be
estimated as depicted in Figure 11.2.
In a situation whereby an inert material is present along
with a pure substance, from the generated thermogram the composition of the
mixture may be derived from the percentage weight variation which takes place
relative to the percentage weight variation observed with the pure compound
(A), by employing the following expression :
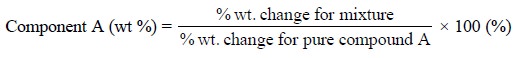
4. APPLICATIONS
The most broadly based application of the
thermogravimetric analysis (TGA) has been visualized and exploited in the
investigation of analytical methods, such as :
(i) Determining
appropriate forms for many elements,
(ii) Screening
and testing of substances which may be used as potential analytical standards
(primary standard), and
(iii) Direct
application of the technique in analytical assays.
A few typical applications of TGA are, namely :
(a) Plateaus
for hydrates are sometimes based on the initial water content (i.e., water of crystalliza-tion). It has
been observed that in humidified air at low heating rates, hydrates usually
give rise to good plateaus.
Example : Dehydration of sodium
tungstate 28-hydrate [Na2WO4.28 H2O (5 : 12)]
Experimental parameters* :
A. Humidified air, 300°C/hour,
B. Humidified air, 150°C/hour,
C. Humidified air 10°C/hour,
D. Room air, 10°C/hour,
Sample weight : 0.5000 g ;
n = Moles water per 5Na2O,
12 WO3
(b) Analysis of
flue-gas scrubber system in environmental analysis.
The flue-gas emitted from a coal-fired-power-plant is subjected
to scrubbing by the aid of wet limestone to get rid of sulphur dioxide (SO2)
as completely as possible. TGA helps in monitoring the system by carrying out
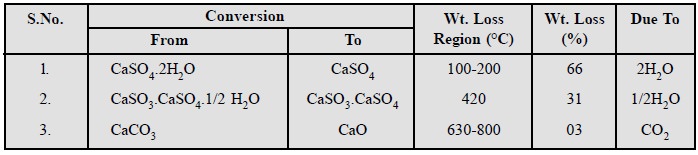
the analysis of the products resulting from the scrubbing process, that mainly
consist of (i) CaCO3 ; (ii) CaSO3 . CaSO3
. 1/4 H2O, and (iii) CaSO4
. 2H2O.
The thermogram obtained from TGA provides the following valuable informations which suggests the decomposition occurring at three distinct stages thereby causing the loss due to two moles of water, half-a-mole of water and one mole of CO2.
(c) The stepwise degradation
of organic polymers has received adequate attention which has broadened the
in-depth knowledge of polymer chemistry. In this specific instance the sample is
either heated under vacuum or in an inert atmosphere (of N2).
(d) The thermogravimetric data
may be employed to evaluate the kinetic parameters of weight varia-tions in
reactions.
Related Topics