Chapter: Pharmaceutical Drug Analysis: Thermoanalytical Analysis
Differential Thermal Analysis (DTA)
DIFFERENTIAL THERMAL ANALYSIS (DTA)
1. THEORY
The difference of temperature between the sample under
estimation and a thermally-inert reference material is continuously recorded as
a function of furnace temperature in differential thermal analysis (DTA). In
actual practice both TGA and DTA are regarded as complementary techniques
whereby information gathered by the usage of one approach is invariably
supplemented and enhanced by the application of the other method. The range of
phenomena measurable during a DTA-run is found to be much larger than in a
TGA-run.
It is pertinent to mention here that in the course of TGA
many vital processes, for instance : crystalli-zation, crystalline transitions,
pure fusion reactions, glass transitions, and solid-state reactions devoid of
vola-tile components might not be indicated as they happen to cause little
change in weight of the sample. TGA invariably describes with ample precision
the stoichiometry related to chemical changes that are indi-cated during DTA by
an endothermal or exothermal duration from the base-line.
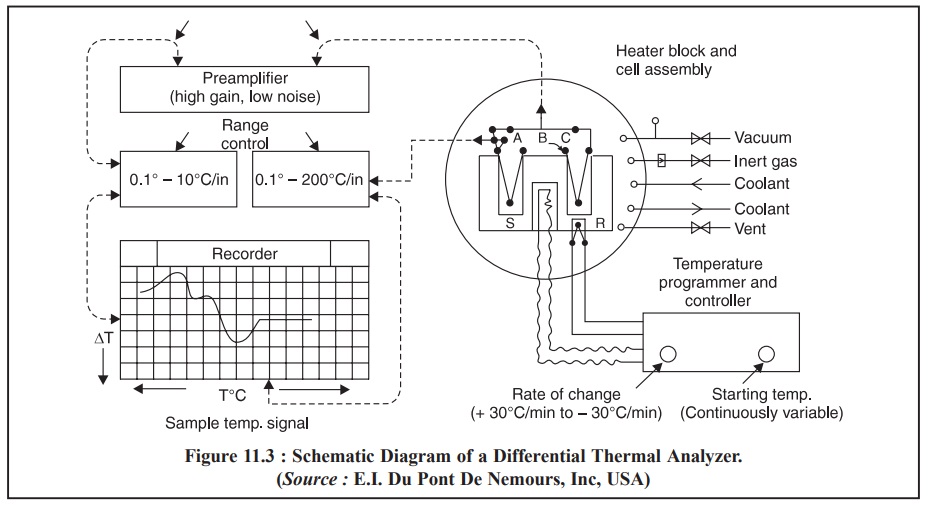
2. INSTRUMENTATION
A differential thermal analyzer is composed of five basic components, namely :
(i) Sample
holder with built-in thermocouple assembly,
(ii)
Flow-control system,
(iii) Furnace
assembly,
(iv)
Preamplifier and Recorder, and
(v) Furnace Power
Programmer and Controller.
A typical commercial differential thermal analyzer is
schematically illustrated in Figure 11.3.
(a)
Thermocouples employed are normally unsheathed Platinum Vs Platinum and Sodium Vs
10% Rhodium. The said two thermocouples help in measuring the difference in
temperature between a sample S and an absolutely inert reference substance R,
as both are subjected to heating in a ceramic or metal block inside a furnace
being operated by a temperature programmer and controller.
(b) The output
of the differential thermocouple is amplified adequately through a high gain,
low-noise preamplifier and subsequently hooked to the recorder, one axis of
which is driven by the block temperature signal and is measured by a third
thermocouple.
(c) Heating/Cooling Device : A sufficient
versatility is achieved by the aid of a pressure-vacuum, high-temperature
electric furnace. An almost constant heating rate is usually achieved by using
a motor-driven variable auto transformer.
Both heating rates and cooling rates may be conveniently
adjusted continuously :
(i) From
0°-30°C/minute by some instruments, and
(ii) From a
choice of several commonly employed heating rates viz., 2°, 4°, 8° and 16°C/minute.
Usual workable sample temperatures are upto : 500°C.
Exceptional maximum temperatures are upto : 1000°C.
(d) Relatively
small sample volumes help in two ways
: first, they make evacuation easy ;
and secondly, they minimize thermal
gradients.
3. METHODOLOGY
(i) Insert a
very thin thermocouple into a disposable sample tube 2 mm in diameter and
containing 0.1-10 mg of sample,
(ii) Another
identical tube is either kept empty or filled with a reference substance, such
as quartz, sand, alumina or alundum powder,
(iii) The two
tubes are simultaneously inserted into the sample block and subsequently heated
(or cooled) at a uniform predetermined programmed rate, and
(iv) DTA—being
a dynamic process, it is extremely important that all aspects of the technique
must be thoroughly standardised so as to obtain reproducible results. A few of
these aspects vital aspects are :
·
Pretreatment of the specimen,
·
Particle size and packing of the specimen,
·
Dilution of the specimen,
·
Nature of the inert diluent,
·
Crystalline substances must be powdered, and sieved
through 100-mesh sieve,
·
For colloidal particles (e.g., clays), micelle-size is very critical, and
·
Either to supress an unwanted reaction (e.g., oxidation), or to explore the
study of a reaction (e.g., gaseous
reaction product)—the atmosphere should be controlled adequately.
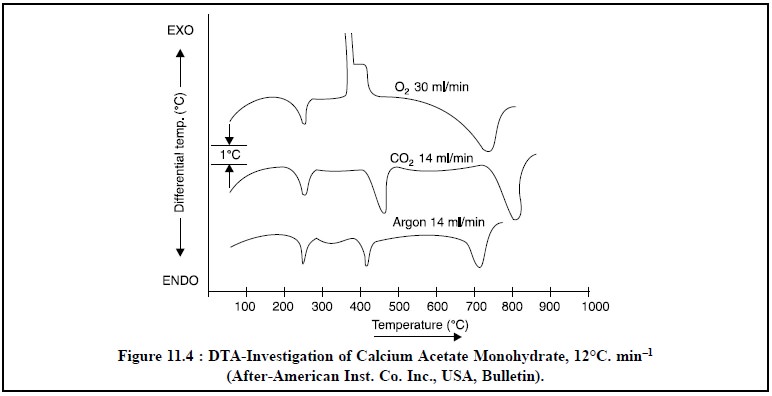
Figure 11.4, depicts the differential thermal analysis
investigation of calcium acetate monohydrate at a uniform programmed heating
rate of 12°C/minute..
The chemical reactions involved in the differentiated
thermal analysis of calcium acetate monohydrate may be expressed as follows :
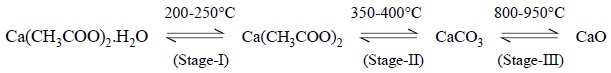
Stage I : The endothermal dehydration of
calcium acetate monohydrate occurs giving rise to the anhydrous salt. It is easily noticed by an endothermal band on DTA
curve between 200°C and 250°C.
Stage II : The anhydrous salt undergoes
endothermal decomposition reaction at 350-400°C resulting into the formation of CaCO3. It has been observed that
this decomposition reaction seems to be almost alike in the presence of either
CO2 or Ar.
Stage III : The decomposition of calcium
carbonate to calcium oxide, which is a function of the partial pressure of the CO2 in
contact with the sample. The endothermal band for the carbonate decomposition
is sharply peaked spread over a relatively narrower temperature range in an
atmosphere of CO2.
4. APPLICATIONS
The various important applications of DTA are :
(i) Rapid
identification of the compositions of mixed clays,
(ii) Studying
the thermal stabilities of inorganic compounds,
(iii)
Critically examining in a specific reaction whether a new compound is actually
formed or the product is nothing but an unreacted original substance, and
(iv) DTA offers a wide spectrum of useful investigations
related to reaction kinetics, polymerization, solvent retention,
phase-transformations, solid-phase reactions and curing or drying properties of
a product.
Related Topics