Chapter: Pharmaceutical Drug Analysis: Diazotization (Sodium Nitrite Titration)
Diazotization (Sodium Nitrite Titration)
DIAZOTIZATION (SODIUM NITRITE TITRATION)
INTRODUCTION
In general, aromatic primary amino moiety (i.e., Ar-NH2), as present in
a host of sulphadrugs viz., succinyl
sulphathiazole, sulphamethoxazole, sulphaphenazole and other potent
pharmaceutical substances, for instance sodium or calcium aminosalicylate,
isocarboxazid, primaquine phosphate, procainamide hydro-chloride, procaine
hydrochloride and dapsone react with sodium nitrite in an acidic medium to
yield the corresponding diazonium salts as expressed below :
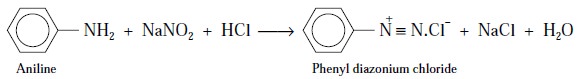
It is interesting to observe here that the above reaction
is absolutely quantitative under experimental parameters. Therefore, it forms
the basis for the estimation of pharmaceutical substances essentially
contain-ing a free primary amino function as already illustrated earlier.
THEORY
Nitrous acid is formed by the interaction of sodium
nitrite and hydrochloric acid as follows :
NaNO2 + HCl ------à NaCl + HNO2
The end-point in the sodium nitrite titration is
determined by the liberation of iodine from iodide which may be expressed by
the following equations :
KI + HCl → HI + KCl
2HI + 2HNO2
→ I2 +
2NO + 2H2O
In other words, the small excess of HNO2
present at the end-point can be detected visually by employ-ing either
starch-iodide paper or paste as an external indicator. Thus, the liberated
iodine reacts with starch to form a blue green colour which is a very sensitive
reaction. Besides, the end-point may also be accomplished electrometrically by
adopting the dead-stop end-point technique, using a pair of platinum electrodes
immersed in the titration liquid.
Related Topics