Chapter: Pharmaceutical Drug Analysis: Diazotization (Sodium Nitrite Titration)
Diazotization (Sodium Nitrite Titration): Assay Methods
ASSAY METHODS
A number of pharmaceutical substances can be assayed by
official methods employing sodium nitrite titrations. A few typical examples
are described below to get an indepth knowledge about sodium nitrite
titrations.
1. PREPARATION OF 0.1 M SODIUM NITRITE SOLUTION
Materials Required : Sodium nitrite : 7.5 g.
Procedure : Weigh accurately 7.5 g of
sodium nitrite and add sufficient DW to produce 1 litre in a 1000 ml volumetric flask.
2. STANDARDIZATION OF 0.1 M SODIUM NITRITE SOLUTIOlN WITH SULPHANILAMIDE
Materials Required : Sulphanilamide (previously
dried at 105°C for 3 hours) : 0.5 g ; hydrochloric acid ( −~
11.5 N) : 20 ml ; 0.1 M sodium nitrite.
Theory : The nitrous acid, generated on
the introduction of sodium nitrite solution into the acidic reaction mixture, reacts with the primary amino group of
sulphanilamide quantitatively, resulting into the formation of an unstable
nitrite that decomposes ultimately with the formation of a diazonium salt. The
diazonium salt thus produced is also unstable, and if the reaction mixture is
not maintained between 5-10°C, it shall undergo decomposition thereby forming phenol
products which may react further with nitrous acid. The reactions involving the
formation of the diazonium salt may be expressed in the following manner :
NaNO2 + HCl → HNO2
+ NaCl
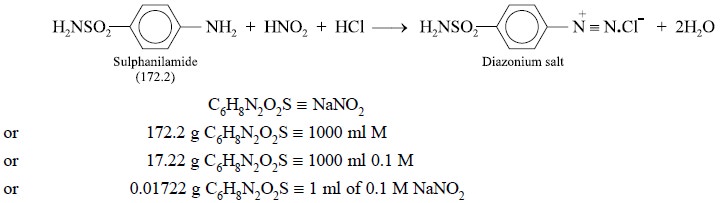
At the equivalence point a slight excess of HNO2
is present which must persist for at least 1 minute. This excess HNO2
may be detected by employing either starch iodide strip or paste and designated
by the following equation :

Procedure : Weigh accurately 0.5 g of
suphanilamide and transfer to a beaker. Add to it 20 ml of hydrochloric acid and 50 ml of DW, stir until dissolved and cool
to 15°C in an ice-bath. Add to it 25 g of crushed ice, and titrate slowly with
sodium nitrite solution, stirring vigorously, until the tip of the glass rod
dipped into the titrated solution immediately produces a distinct blue ring on
being touched to starch-iodide paper. The titration is supposed to be complete
when the end-point is deducible after the resulting mixture has been allowed to
stand for 1 minute. Each 0.01722 g of sulphanilamide is equivalent to 1 ml of
0.1 N sodium nitrite.
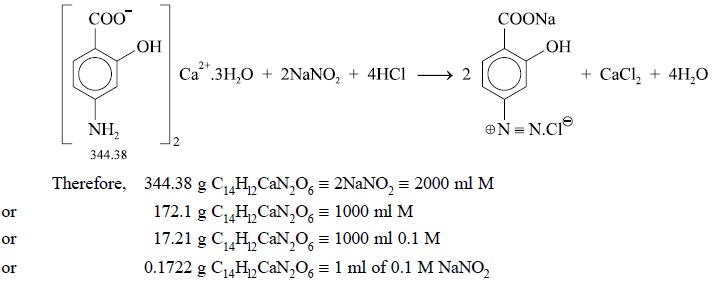
3. CALCIUM AMINOSALICYLATE
Materials Required : Calcium aminosalicylate : 0.5
g ; hydrochloric acid ( −~ 11.5
N) : 10.0 ml ; potassium bromide : 1.0 g ; 0.1 M sodium nitrite ; starch-iodide
paper.
Theory : The assay of calcium
aminosalicylate is based upon the reaction designated by the following equation :
Procedure : Weigh accurately about 0.5 g
of calcium aminosalicylate, into a funnel placed in the mouth of a 250 ml volumetric flask. Wash through with 10 ml of
hydrochloric acid and enough DW to dissolve, add 1.0 g potassium bromide and
make up the volume upto 250 ml mark. Pipette 50 ml into a conical flask, cool
to below 15°C (in ice-bath) and titrate gradually with 0.1 M sodium nitrite
solution while shaking the contents of the flask vigorously and continuously
until a distinct blue colour is achieved when a drop of the titrated solution
is placed on a starch-iodide paper 5 minutes after the last addition of the 0.1
M NaNO2 solution. Care must be taken to add NaNO2
solution at the rate of 0.1 ml near the end of the titration. Each ml of 0.1 M
sodium nitrite is equivalent to 0.01722 g of C14Hl2CaN2O6.
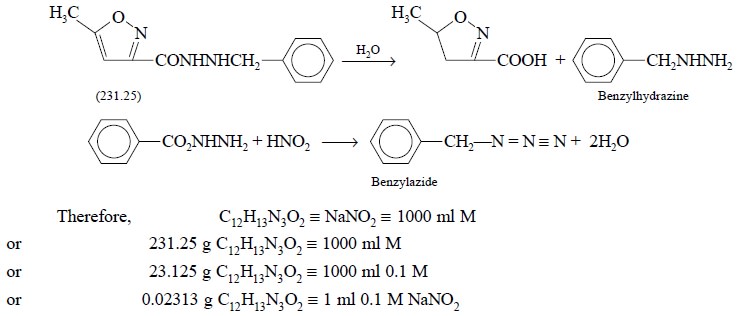
4. ISOCARBOXAZID
Materials Required : Isocarboxazid : 0.5 g ;
glacial acetic acid (99% w/w or 17.5 N) : 20.0 ml ; hydrochloric acid ( ~− 11.5 N) : 20.0 ml ; 0.1 M sodium nitrite ;
starch-iodide paper.
Theory : The estimation is based on the
fact that isocarboxazid undergoes rapid cleavage in acidic medium to produce benzylhydrazine. The latter reacts
quantitatively with nitrous acid (NaNO2 and HCl) to give rise to
benzylazide. The various reactions involved are expressed as follows :
Procedure : Weigh accurately about 0.5 g
of isocarboxazid and dissolve it in 20 ml of glacial acetic acid. Add to it 20 ml of hydrochloric acid and 50 ml of DW. Cool
to about 15°C in an ice-bath and titrate slowly with 0.1 M NaNO2
while shaking vigorously and continuously until a distinct blue colour is
obtained on a starch-iodide paper that lasts for 5 minutes after the final
addition of the 0.1 M NaNO2 solution to the titrated solution. Add
NaNO2 solution very carefully at the rate of 0.1 ml at a time as the
end-point is approached. Each mole of 0.1 M sodium nitrite is equivalent to
0.02313 g of C12H13N3O2 .
5. PHTHALYLSULPHATHIAZOLE
Materials Required : Phthalylsulphathiazole : 0.5 g
; sodium hydroxide solution (20% w/v in water) : 10.0 ml ; hydrochloric acid (
~− 11.5 N) : 20.0 ml
; 0.1 M sodium nitrite ; starch-iodide paper.
Theory : The assay is based upon the
reactions designated by the following equations :
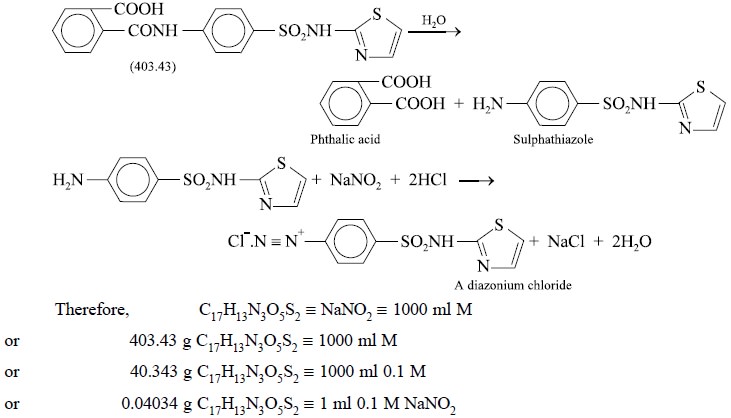
Phthalylsulphathiazole undergoes hydrolysis to give
phthalic acid and sulphathizole. The latter reacts with nitrous acid to yield
the corresponding diazonium salt quantitatively.
Procedure : Weigh accurately about 0.5 g
of phthalylsulphathiazole and heat on a water-bath for 2 hours after the addition of 10.0 ml of sodium hydroxide solution.
Cool the contents of the flask to 15°C in an ice-bath, add to it 10.0 ml of
water and 20.0 ml of hydrochloric acid and carry out the titration slowly with
0.1 M sodium nitrite solution. The contents of the flask are shaken thoroughly
and continuously until a distinctly visible blue colour is obtained when a drop
of the titrated solution is placed on a starch-iodide paper 5 minutes after the
last addition of the 0.1 M NaNO2 solution. Towards the approach of
the end-point the addition of NaNO2 solution must be at the rate of
0.1 ml. Each ml of 0.1 M sodium nitrite is equivalent to 0.04034 g of C17H13N3O5S2.
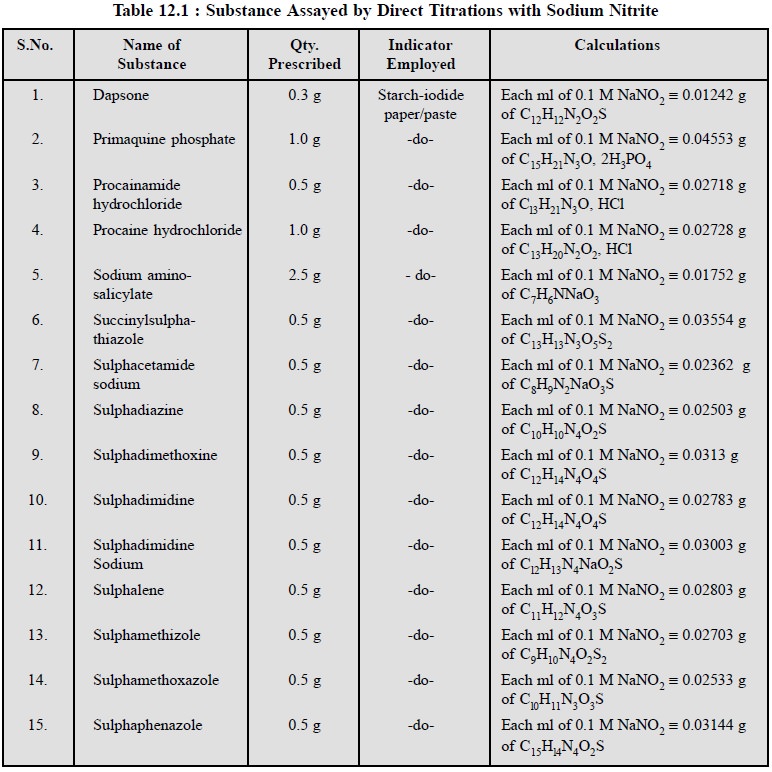
6. COGNATE ASSAYS
A plethora of pharmaceutical substances that can be
assayed by the help of sodium nitrite titrations are mentioned in Table 12.1.
Related Topics