Chapter: Pharmaceutical Drug Analysis: Permanganate, Dichromate and Ceric Sulphate Titration Methods
Theory - Permanganate, Dichromate and Ceric Sulphate Titration Methods
THEORY
As a number of elements are capable of exhibiting more
than one oxidation state, hence volumetric titration methods based on redox
reactions are usually employed widely.
The phenomenon of oxidation may be explained in the
following manner :
(i)
addition of oxygen :
Example : 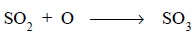
(ii)
removal of hydrogen :
Example : 
(iii)
enhancement in the ratio of electronegative to the electropositive portion of
the molecule :
Examples :
In the same vein, the process of reduction may also be
explained as stated below :
(i) addition of
hydrogen :
Example :
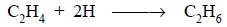
(ii) removal of
oxygen :
Example :

(iii)
enhancement in the ratio of electropositive to electronegative portion of the
molecule :
Example : [same as under oxidation (iii) above]
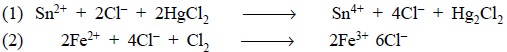
It is quite evident from the above cited examples that
reduction need not always imply a reaction involving hydrogen, since HgCl2
is reduced to Hg2Cl2, and that oxidation may not
essentially suggest a reaction involving oxygen, since Fe2+ is
oxidized by Cl2 to Fe3+. It is, therefore, pertinent to
observe here that whenever one entity undergoes oxidation, definitely some
other entity undergoes reduction correspondingly and vice-versa. In other words, there always exists a transfer of
electrons in oxidation-reduction reactions, because in every such reaction the
charge gained or lost by one substance must essentially be lost or gained by
another.
A reducing agent
is the reactant that loses electrons in an oxidation-reduction reaction :
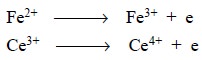
Thus, the reactant containing a constituent atom or atoms
are converted to a higher state of oxidation.
An oxidizing agent is the reactant that gains electrons
in an oxidation-reduction reaction :
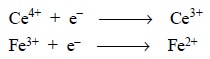
Thus, the reactant containing a constituent atom or atoms
are converted to a lower state of oxidation.
The quantitative measurement of one of the reactants may
be accomplished by the reaction derived from the combination of oxidizing and
reducing agents, for instance

and hence, ferrous sulphate can be estimated
quantitatively by its reaction with ceric sulphate.
Related Topics