Chapter: Pharmaceutical Drug Analysis: Permanganate, Dichromate and Ceric Sulphate Titration Methods
Dichromate Titration Methods
DICHROMATE METHODS
Potassium dischromate (K2Cr2O7)
is a strong oxidizing agent, quite comparable to KMnO4 that nor-mally
shows only one pertinent reduced oxidation state : Thus, chemically we have :
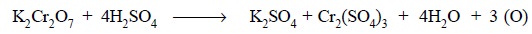
Ionically we have :
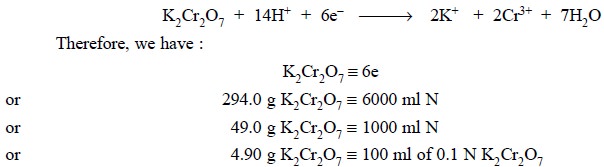
Potassium dichromate exhibits much greater stability in
aqueous solution in comparison to potassium permanganate. Potassium dichromate
possesses an inherent oranage colour that is not intense enough to serve its
own end-point signal, specifically in the presence of the green Cr3+
ion, which is supposed to be present at the end-point. Hence, redox indicators
are usually employed to locate the exact end-point e.g., barium diphenylamine sulphonate.
1. Preparation of 0.1 N Potassium Dichromate Solution
Materials Required : Potassium dichromate : 4.930
g.
Procedure : Weigh accurately 4.93 g of
potassium dichromate previously powdered and dried at 20°C for 4 hours and dissolve in sufficient DW to produce 1 litre
in a volumetric flask.
Note : Potassium dichromate
can be obtained as a primary standard reagent and hence, standard solu-tions
may be prepared determinately and stored for long periods of time.
Equations : Chemically we have :
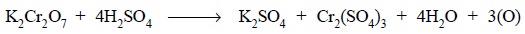
Ionically we have :

From this equation it follows that the equivalent weight
of potassium dichromate is 1/6th of the molecular weight i.e., 294.22/6 or 49.03 g.
2. Standardization of 0.1 N Potassium Dichromate Solution
It can be achieved by following these steps, namely :
(a) Preparation of Standard Solution of Mohr’s
Salt FeSO4(NH4)2.SO4.6H2O
:
Materials Required : Mohr’s salt : 4.9 g ; dilute
sulphuric acid (1 in 3, approx. 9 N) : 20 ml.
Procedure : Weigh accurately about 4.9 g
of pure sample of Mohr’s salt and transfer it to a 250 ml volumetric flask. Add 20 ml of dilute sulphuric acid and make up
the volume to the mark with DW and finally mix the contents of the flask
thoroughly.
Calculations : The quantity of Mohr’s salt
required for 250 ml of the solution having a normality of 0.05 N can be calculated as follows :
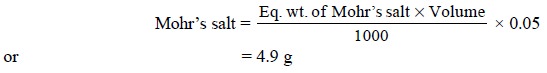
(b) Standardization of 0.1 N K2Cr2O7
Solution :
Materials Required : Standard solution of Mohr’s
salt (0.05 N) : 250 ml, sulphuric acid (2 N) : 20 ml ; potassium dichromate
solution (0.1 N) : 1 litre.
Procedure : Transfer 20 ml of the primary
standard solution (Mohr’s salt) to the titration flask and add 20 ml of 2 N sulphuric acid. Take the potassium dichromate
solution in the burette. Put drops of freshly prepared potassium ferricyanide,
K3[Fe(CN)6], solution in the grooves of a porcelain tile.
Now, proceed with the titration of Mohr’s salt solution against K2Cr2O7
solution. Transfer drops of the titrated solution by means of a glass rod and
mix with drops of the indicator, already taken in the groove-tile.
Alternatively, pre-soaked and dried filter paper with K3[Fe(CN)6]
solution can also be used in place of the groove-tile method.
In order to arrive at the exact end-point the above
titration may be carried out at three
stages, namely :
Stage 1 : Spot tests are carried out at
intervals of 1-2 ml until a blue colour is no longer produced with K3[Fe(CN)6],
which provides an altogether rough estimate of the K2Cr2O7
solution required for the titration,
Stage 2 : Spot tests are only performed
near the approach of the end of titration at intervals of 0.1-0.2 ml, and
Stage 3 : Spot tests are finally done only
at the end-point.
The above sequential steps give fairly accurate results
because the error caused by the removal of part of the solution for the spot
tests is made negligibly small. However, the titration is repeated to get a set
of concordant readings.
By applying the relationship between N1V1
(K2Cr2O7) and N2V2
(Mohr’s salt), the normality of the former may be calculated.
2.1. Iron Ore
Materials Required : Iron ore : 0. 1 g ;
hydrochloric acid (conc.) : 15 ml ; diphenylamine (1% w/v in conc. H2SO4) ;
zinc metal (granulated) : 4 g ; ammonium thiocyanate solution (0.1% in water) ;
mixture of sulphuric acid and phosphoric acid [dissolve 15 ml of H2SO4
(sp. gr. 1.84) in 50 ml of DW, cool and add 15 ml of H3PO4
(sp. gr. 1.70) and make the volume to 100 ml with DW] : 25 ml.
Procedure :
(a) Preparation of Standard K2Cr2O7
Solution : Instead of using
solutions having definite normal-ity, routine industrial laboratories make use
of ‘emperical solution’ which is
normally expressed in terms of ‘titer for
the substance determined’. For this assay, let us prepare an emperical K2Cr2O7
solution (250 ml) of such a concentration that 1 ml of the same exactly
correspond to 0.0025 g Fe.
Calculations :
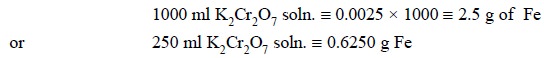
By Law of Equivalence, we have :
1 gram-equivalent of K2Cr2O7
(49.03 g) ≡ 1 gram-equivalent of Fe (55.85 g)
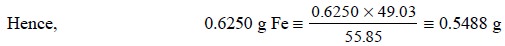
Therefore, weigh accurately 0.5488 g of pure K2Cr2O7
and transfer it quantitatively into a 250 ml volumetric flask, dissolve in DW,
make up the volume and mix thoroughly.
Hence, the ‘iron
titer’ of this solution is :
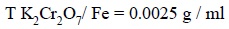
(b) Preparation of Ore Solution : Weigh
accurately 0.1 g of powdered and dried ore on a clean watch glass and transfer
it quantitatively into a 100 ml-volumetric flask. Add 15 ml of concen-trated
hydrochloric acid, warm the contents of the flask carefully over a sand-bath
until most of the dark grains of ore get dissolved completely and only a
whitish silica precipitate settles at the bottom of the flask.
(c) Reduction of Fe3+ to F2+ in the Ore Solution : Introduce carefully a few pieces of
granulated pure zinc metal into the flask, place a funnel in the neck of the
flask to avoid splashes and boil the solution gently until the yellow colour
has disappeared completely, thereby ascertaining that com-plete reduction of Fe3+
to Fe2+ is affected.
Note : It may be further
confirmed by doing a spot test with NH4CNS solution which only shows
a blood-red colour with Fe3+.
The contents of the flask is cooled, filtered through
cotton wool, washings done with DW and the filtrate diluted to about 350 ml
with DW. This dilution is a must so as to avoid any interference caused by its
inherent green colour with the estimation of the equivalence point in the
titration as per the following chemical reaction :
K2Cr2O7 +
6FeCl2 + 14HCl → 2CrCl3 +
2KCl + 6FeCl3 + 7H2O
(d) Final Titration : The 350 ml solution
obtained in (c) above is now
quantitatively titrated against K2Cr2O7
solution employing diphenylamine as an internal indicator. Add 25 ml of a
mixture of sulphuric acid and phosphoric acid to the solution along with 2
drops of diphenylamine indicator and titrate the solution with K2Cr2O7
solution carefully, by adding small lots at intervals with constant shaking,
until a persistant blue-violet colour appears.
Note : (a) The acidity of the
solution must be maintained fairly high which can be achieved by adding
orthophosphoric acid, H3PO4,
(b) The quantity of diphenylamine must not
exceed 2 drops by virtue of the fact that at higher concentration with lower acidity during very slow titration, the
indicator undergoes an altogether different type of chemical change that
ultimately gives a green colour instead of the desired blue-violet colour.
(e) Calculations : Multiply the number of
millilitres of K2Cr2O7 Solution consumed in the
titration by the ‘iron titer’ and therefrom determine the amount of iron
present in the sample. Finally, the percentage of iron present in the ore may
be calculated.
Related Topics