Chapter: Pharmaceutical Drug Analysis: Permanganate, Dichromate and Ceric Sulphate Titration Methods
Permanganate Titration Methods
PERMANGANATE METHODS
The vital application of potassium permanganate as a potential oxidizing agent in an acidic medium mainly rests on the reactions designated by the following equations :
Chemically we have :
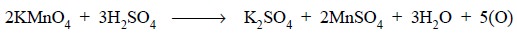
Ionically we have :
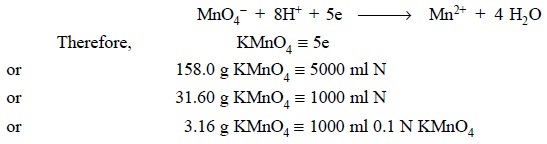
1. Preparation of 0.1 N Potassium Permanganate Solution
Materials Required : Potassium permanganate : 3.5 g.
Procedure : Weigh accurately about 3.2 g of potassium permanganate on a watch-glass. Transfer the contents to a 250 ml beaker containing cold water and stir vigorously with a glass rod to effect rapid dissolution. Decant the solution through a small plug of glass wool supported by a funnel, into a 1 litre volumetric flask thereby leaving the undissolved residues in the beaker. Add more DW to the beaker and repeat the above process till all the potassium permanganate gets dissolved. Finally make up the volume to the graduated mark and shake well so as to effect uniform mixing.
Note : (i) KMnO4 must be weighed on a watch-glass and not on any kind of paper since cellu-lose fibers are corrosively attacked by it,
(ii) Likewise, filtration of KMnO4 solution must be done though cleaned glass wool and not cotton wool, and
(iii) Avoid heat in the preparation of KMnO4 solution because traces of grease or other pos-sible contaminants on the glass vessels used can catalyse its decomposition.
2. Standardization of 0.1 N Potassium Permanganate Solution
Materials Required : Oxalic acid : 6.3 g ; sulphuric acid concentrated : 5 ml.
Theory : The standardization of potassium permanganate solution is based upon the following equations :
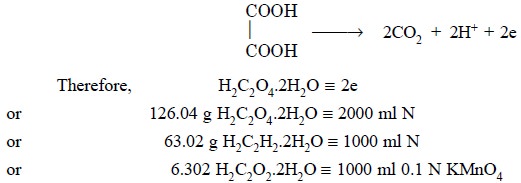
Procedure : Weigh accurately about 6.3 g of pure oxalic acid (AnalaR-Grade) into a 1 litre volumet-ric flask, dissolve in suffcient DW and make up the volume upto the mark. Pipette out 25 ml of this solution, add to it 5 ml of concentrated sulphuric acid along the side of the flask, swirl the contents carefully and warm upto 70°C. Titrate this against the potassium permanganate solution from the burette till the pink colour persists for about 20 seconds.
Precautions :
(i) Sufficient acid must be present, otherwise formation of a brown colour during titration may be observed,
(ii) Similar brown colouration can also be observed by using too high a temperature or by using a dirty flask, and
(i) To avoid such anomalies always rinse the flask with solution of H2O2 and dilute H2SO4 before performing the titrations.
3. Direct Titration Methods
Hydrogen peroxide solution and potassium bromide are two pharmaceutical substances that may be estimated by employing 0.1 N potassium permanganate solution and adopting the direct titration method.
3.1. Hydrogen Peroxide Solution
Materials Required : Hydrogen peroxide solution : 10 ml ; 5 N sulphuric acid : 5 ml ; 0.1 N potas-sium permanganate.
Procedure : Dilute 10 ml of hydrogen peroxide solution to 250 ml with DW in a volumetric flask. To 25.0 ml of this solution add 5 ml of 5 N sulphuric acid and titrate with 0.1 N KMnO4 to a permanent pink end-point. Each ml of 0.1 N potassium permanganate is equivalent to 0.001701 g of H2O2.
Equations :
Chemically, we have :

Ionically we have :
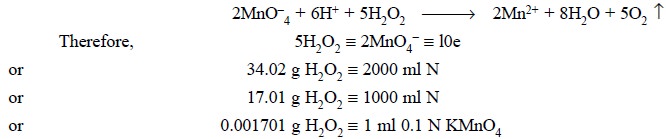
Calculations : (For % w/v of H2O2)
The ‘volume strength’ of the hydrogen peroxide solution is the number of ml of oxygen at NTP* which may be produced by the complete thermal decomposition of 1 ml of H2O2 solution. Hence, decompo-sition takes place as designated by the following equation :
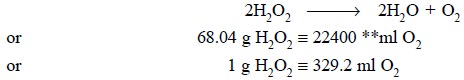
The IP limit of H2O2 solution is 5-7% w/v.
Now, let us consider a sample which contains 6.25 per cent w/v H2O2 :

3.2. Potassium Bromide
Materials Required : Potassium bromide : 1.2 g ; sulphuric acid (36 N) ; 10 ml ; 0.1 N KMnO4.
Procedure : Weigh accurately about 1.2 g of potassium bromide and dissolve in DW and make up the volume to 1 litre mark with water in a volumetric flask. To 10.0 ml of the solution, add 100 ml of DW and 10 ml of (36 N) sulphuric acid along the side of the flask and a few glass beads (to avoid bumping of solution). Heat to boiling and while the solution is still boiling, titrate with 0.1 N KMnO4 added dropwise until the pink colour just persists. Each ml of 0.1 N KMnO4 is equivalent to 0.01190 g of KBr.
Equations :
The Br– is oxidised to bromine by acidified KMnO4, thus :

4. Indirect Titration Methods
In the indirect method of permanganate oxidation certain compounds are first converted by means of chemical reactions to an equivalent amount of oxalate which is then subsequently oxidized quantitatively by permanganate.
4.1. Assay of Cherry Juice for Malic Acid
In this particular assay the malic acid present in the cherry juice is estimated by the following three steps sequentially :
Step 1 : Conversion of malic acid to an equivalent amount of calcium salt,
Step 2 : Conversion of calcium salt to corresponding insoluble calcium oxalate, and
Step 3 : Liberation of oxalate and subsequent oxidation with permanganate.
Materials Required : Cherry juice : 10 ml ; calcium carbonate : 1.0 g ; ammonia TS : 1 ml ; ammonium oxalate TS : 15 ml ; diluted ammonia (1 in 49) : 25 ml ; diluted sulphuric acid (1 in 3 ; approximately 9 N) : 30 ml ; potassium permanganate 0.1 N.
Procedure : Place 10 ml of precisely measured cherry juice in a 125 ml flask and add to it 1 g of calcium carbonate. Heat the contents on a water-bath for 15 minutes while swirling periodically and filter. Wash the filter 2 to 3 times with 5 ml portions of DW. Add to the combined filtrate and washings 1 ml of ammonia TS followed by 15 ml of ammonium oxalate TS. Warm the contents on a water-bath for 15 minutes, filter through filter paper and wash the filter with 5 ml portions of a solution previously made by mixing 1 ml of ammonia TS with 49 ml of DW. Perforate the filter paper and wash the precipitate into the same flask with hot DW and followed by 30 ml of diluted sulphuric acid. The resulting solution is heated to 80°C and finally titrated with 0.1 N KMnO4. Each ml of 0.1 N KMnO4 is equivalent to 6.704 g of C4H6O5.
Equations : Malic acid first reacts with CaCO3 to yield the soluble calcium malate that goes into the filtrate, whereas the insoluble calcium carbonate is filtered off and rejected. Thus,

The interaction between calcium malate and ammonium oxalate results into an equivalent quantity of calcium oxalate by displacement mechanism which is subsequently precipitated :

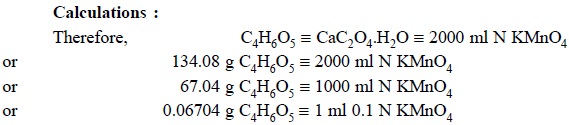
5. Residual Titration Methods
The residual titration method for pharmaceutical substances using potassium permanganate solution are mainly of two categories, namely :
(i) titration wherein an excess of standard oxalic acid is added to the substance and then the excess of oxalic acid is back titrated with KMnO4, and
(ii) titration wherein an excess of standard KMnO4 solution is used to oxidize the product, and then the amount in excess is estimated by reduction with either :
(a) excess ferrous ammonium sulphate and back titrated with more of standard KMnO4, or
(b) excess standard oxalic acid.
5A. Assay of Sodium Nitrite
Materials Required : Sodium nitrite : 1.0 g ; 0.1 N potassium permanganate : 50 ml ; sulphuric acid (conc.) : 5 ml ; 0.1 N oxalic acid.
Procedure : Weigh accurately about 1 g of sodium nitrite and dissolve it in DW to make 100 ml in a volumetric flask. Transfer 10 ml of this solution into a mixture of 50 ml of 0.1 N KMnO4, 100 ml of water and add 5 ml of sulphuric acid along the side of the flask. Heat the contents to 40°C, allow it to stand for 5 minutes and add 25 ml of 0.1 N oxalic acid. Warm the resulting mixture to about 80°C on a steam-bath and titrate with 0.1 N KMnO4 solution. Each ml of 0.1 N potassium permanganate is equivalent to 3.450 mg of NaNO2.
Precautions : While adding NaNO2 solution
(i) Care should be taken to immerse the tip of the pipette beneath the surface of the permanganate mixture, otherwise the nitrous acid (volatile) generated by NaNO2 and H2SO4, would be lost, and
(ii) Oxidation of nitrous acid (HNO2) to nitric acid (HNO3) takes place sluggishly at ambient temperature and hence, it is necessary to warm it upto 40°C for 5 minutes to expedite completion of reaction.
Examples : Chemically we have :
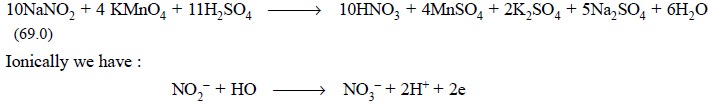
i.e., each molecule of sodium nitrite loses two electrons.
Calculations :
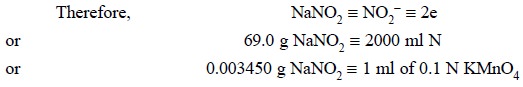
Related Topics