Chapter: Medical Physiology: Adrenocortical Hormones
Synthesis and Secretion of Adrenocortical Hormones
Synthesis and Secretion of Adrenocortical Hormones
The Adrenal Cortex Has Three Distinct Layers. Figure 77–1 shows that the adrenalcortex is composed of three relatively distinct layers:
1. The zona glomerulosa, a thin layer of cells that lies just underneath the capsule, constitutes about 15 per cent of the adrenal cortex. These cells are the only ones in the adrenal gland capable of secreting significant amounts of aldosterone because they contain the enzyme aldosterone synthase, which is necessary for synthesis of aldosterone. The secretion of these cells is controlled mainly by the extracellular fluid concentrations of angiotensinII and potassium, both of which stimulate aldosterone secretion.
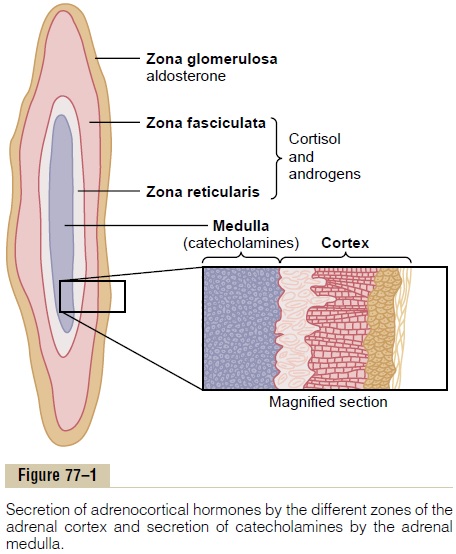
2. The zona fasciculata, the middle and widest layer, constitutes about 75 per cent of the adrenal cortex and secretes the glucocorticoids cortisol and corticosterone, as well as small amounts of adrenal androgens and estrogens. The secretionof these cells is controlled in large part by the hypothalamic-pituitary axis via adrenocorticotropic hormone (ACTH).
3. The zona reticularis, the deep layer of the cortex, secretes the adrenal androgens dehydroepiandrosterone (DHEA) andandrostenedione, as well as small amounts ofestrogens and some glucocorticoids. ACTH also regulates secretion of these cells, although other factors such as cortical androgen-stimulatinghormone, released from the pituitary, may also beinvolved. The mechanisms for controlling adrenal androgen production, however, are not nearly as well understood as those for glucocorticoids and mineralocorticoids.
Aldosterone and cortisol secretion are regulated by independent mechanisms. Factors such as angiotensin II that specifically increase the output of aldosterone and cause hypertrophy of the zona glomerulosa have no effect on the other two zones. Similarly, factors such as ACTH that increase secretion of cortisol and adrenal androgens and cause hypertrophy of the zona fasciculata and zona reticularis have little or no effect on the zona glomerulosa.
Adrenocortical Hormones Are Steroids Derived from Cholesterol.
All human steroid hormones, including those produced by the adrenal cortex, are synthesized from cholesterol. Although the cells of the adrenal cortex can synthesize de novo small amounts of cholesterol from acetate, approximately 80 per cent of the cholesterol used for steroid synthesis is provided by low-density lipoproteins (LDL) in the circulating plasma. The LDLs, which have high concentrations of cholesterol, diffuse from the plasma into the interstitial fluid and attach to specific receptors contained in structures called coated pits on the adrenocortical cell membranes. The coated pits are then internalized by endocytosis, forming vesicles that eventually fuse with cell lysosomes and release choles-terol that can be used to synthesize adrenal steroid hormones.
Transport of cholesterol into the adrenal cells is reg-ulated by feedback mechanisms that can markedly alter the amount available for steroid synthesis. For example, ACTH, which stimulates adrenal steroid synthesis, increases the number of adrenocortical cell receptors for LDL, as well as the activity of enzymes that liberate cholesterol from LDL.
Once the cholesterol enters the cell, it is delivered to the mitochondria, where it is cleaved by the enzyme cholesterol desmolaseto form pregnenolone; this is therate-limiting step in the eventual formation of adrenal steroids (Figure 77–2). In all three zones of the adrenal cortex, this initial step in steroid synthesis is stimulated by the different factors that control secretion of the major hormone products aldosterone and cortisol. For example, both ACTH, which stimulates cortisol secre-tion, and angiotensin II, which stimulates aldosterone secretion, increase the conversion of cholesterol to pregnenolone.
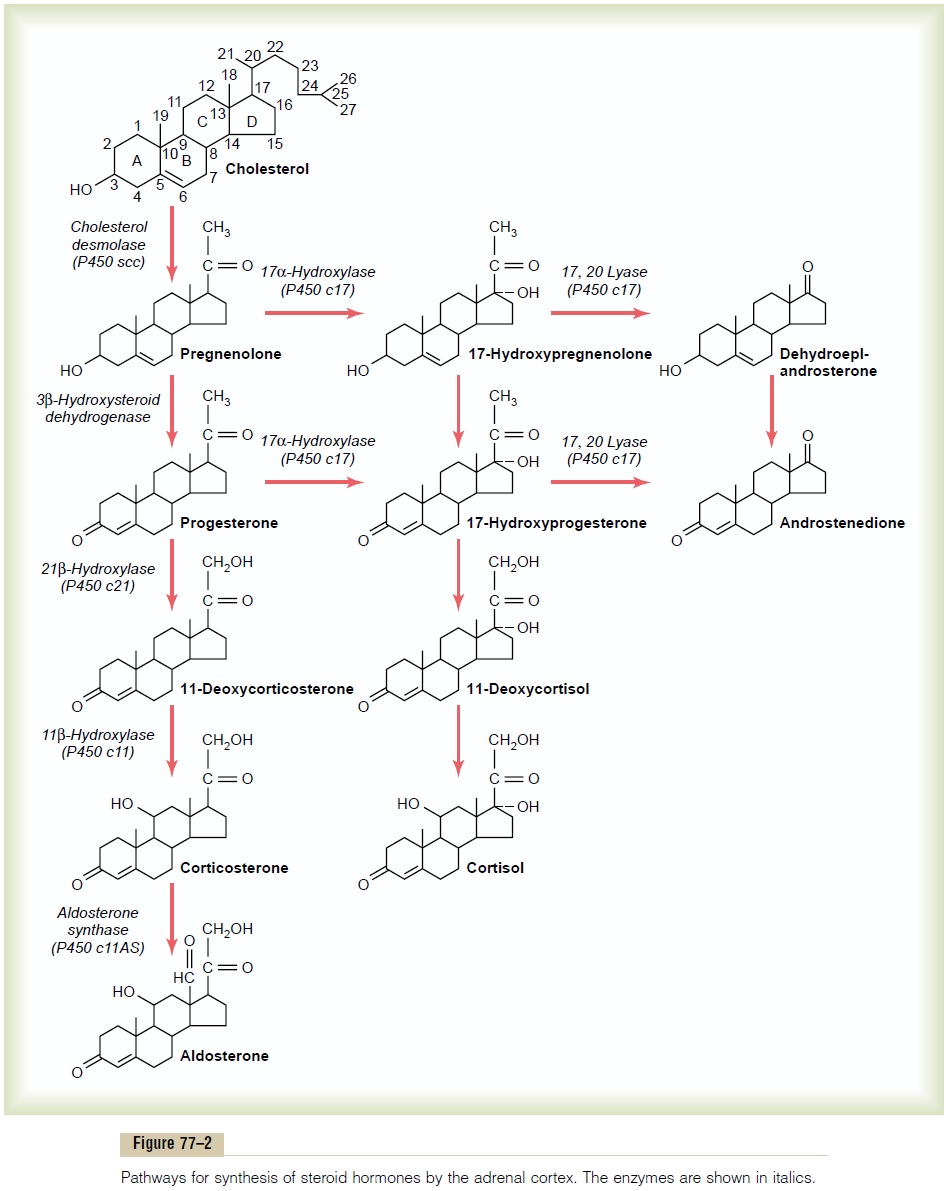
Synthetic Pathways for Adrenal Steroids. Figure 77–2 givesthe principal steps in the formation of the important steroid products of the adrenal cortex: aldosterone, cor-tisol, and the androgens. Essentially all these steps occur in two of the organelles of the cell, themitochondria and the endoplasmic reticulum, some steps occurring in one of these organelles and some in the other. Each step is catalyzed by a specific enzyme system. A change in even a single enzyme in the schema can cause vastly differ-ent types and relative proportions of hormones to be formed. For example, very large quantities of masculin-izing sex hormones or other steroid compounds not normally present in the blood can occur with altered activity of only one of the enzymes in this pathway.
The chemical formulas of aldosterone and cortisol, which are the most important mineralocorticoid and glucocorticoid hormones, respectively, are shown in Figure 77–2. Cortisol has a keto-oxygen on carbon number 3 and is hydroxylated at carbon numbers 11 and 21. The mineralocorticoid aldosterone has an oxygen atom bound at the number 18 carbon.
In addition to aldosterone and cortisol, other steroids having glucocorticoid or mineralocorticoid activities, or both, are normally secreted in small amounts by the adrenal cortex. And several additional potent steroid hormones not normally formed in the adrenal glands have been synthesized and are used in various forms of therapy. Some of the more important of the corticos-teroid hormones, including the synthetic ones, are the following as summarized in Table 77–1:
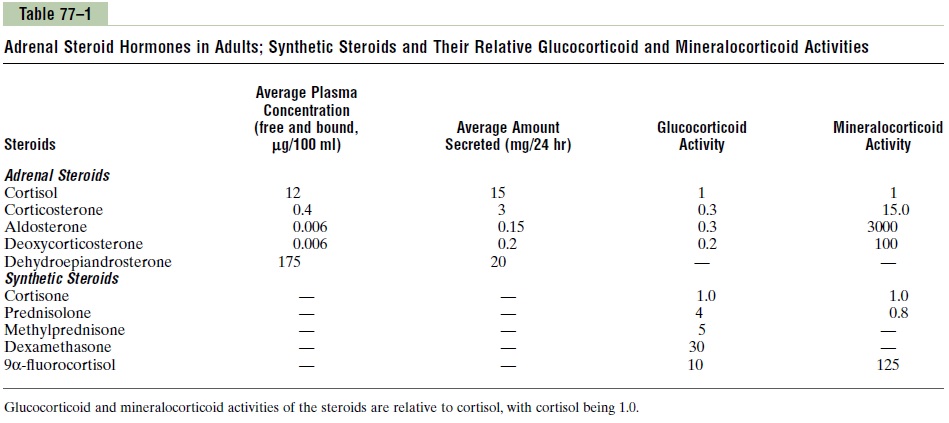
Mineralocorticoids
• Aldosterone (very potent, accounts for about 90 per cent of all mineralocorticoid activity)
• Desoxycorticosterone (1/30 as potent as aldosterone, but very small quantities secreted)
• Corticosterone (slight mineralocorticoid activity)9a-Fluorocortisol (synthetic, slightly more potent than aldosterone)
• Cortisol (very slight mineralocorticoid activity, but large quantity secreted)
• Cortisone (synthetic, slight mineralocorticoid activity)
Glucocorticoids
• Cortisol (very potent, accounts for about 95 per cent of all glucocorticoid activity)
• Corticosterone (provides about 4 per cent of total glucocorticoid activity, but much less potent than cortisol)
• Cortisone (synthetic, almost as potent as cortisol)
• Prednisone (synthetic, four times as potent as cortisol)
• Methylprednisone (synthetic, five times as potent as cortisol)
• Dexamethasone (synthetic, 30 times as potent as cortisol)
It is clear from this list that some of these hormones have both glucocorticoid and mineralocorticoid activi-ties. It is especially significant that cortisol has a smallamount of mineralocorticoid activity, because some syn-dromes of excess cortisol secretion can cause significant mineralocorticoid effects, along with its much more potent glucocorticoid effects.
The intense glucocorticoid activity of the synthetic hormone dexamethasone, which has almost zero min-eralocorticoid activity, makes this an especially impor-tant drug for stimulating specific glucocorticoid activity.
Adrenocortical Hormones Are Bound to Plasma Proteins.
Approximately 90 to 95 per cent of the cortisol in the plasma binds to plasma proteins, especially a globulin called cortisol-binding globulin or transcortin and, to a lesser extent, to albumin. This high degree of binding to plasma proteins slows the elimination of cortisol from the plasma; therefore, cortisol has a relatively long half-life of 60 to 90 minutes. Only about 60 per cent of cir-culating aldosterone combines with the plasma proteins, so that about 40 per cent is in the free form; as a result, aldosterone has a relatively short half-life of about 20 minutes. In both the combined and free forms, the hor-mones are transported throughout the extracellular fluid compartment.
Binding of adrenal steroids to the plasma proteins may serve as a reservoir to lessen rapid fluctuations in free hormone concentrations, as would occur, for example, with cortisol during brief periods of stress and episodic secretion of ACTH. This reservoir function may also help to ensure a relatively uniform distribu-tion of the adrenal hormones to the tissues.
Adrenocortical Hormones Are Metabolized in the Liver. Theadrenal steroids are degraded mainly in the liver and conjugated especially to glucuronic acid and, to a lesser extent, sulfates. These substances are inactive and do not have mineralocorticoid or glucocorticoid activity. About 25 per cent of these conjugates are excreted in the bile and then in the feces. The remaining conjugates formed by the liver enter the circulation but are not bound to plasma proteins, are highly soluble in the plasma, and are therefore filtered readily by the kidneys and excreted in the urine. Diseases of the liver markedly depress the rate of inactivation of adrenocortical hor-mones, and kidney diseases reduce the excretion of the inactive conjugates.
The normal concentration of aldosterone in blood is about 6 nanograms (6 billionths of a gram) per 100 ml, and the average secretory rate is approximately 150 µg/day (0.15 mg/day).
The concentration of cortisol in the blood averages 12 µg/100 ml, and the secretory rate averages 15 to 20 mg/day.
Related Topics