Chapter: Pharmaceutical Drug Analysis: Gravimetric Analysis
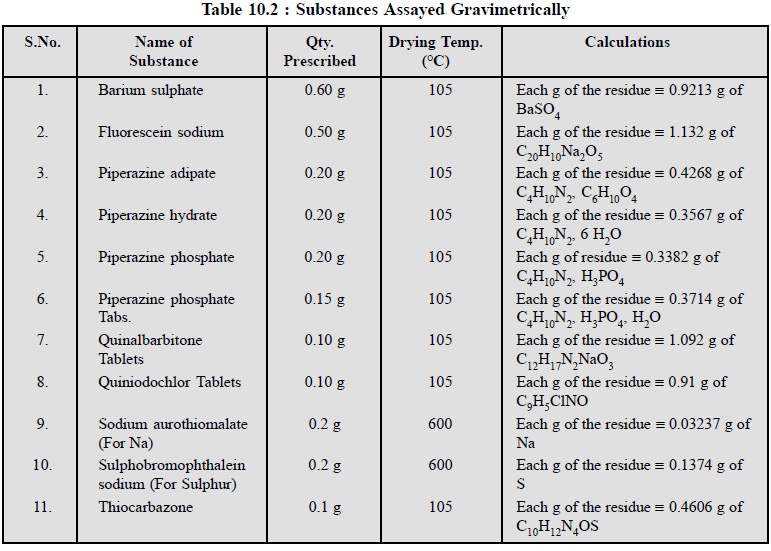
Substances Assayed Gravimetrically
SUBSTANCES ASSAYED GRAVIMETRICALLY
A good number of pharmaceutical substances may be
determined gravimetrically by obtaining their respective difficultly soluble
salts as precipitates, weighing to a constant weight and finding the percentage
purity of the substance in question.
A few typical examples are cited below so as to expatiate
the procedure as well as the theoretical aspects.
1. Sodium Chloride
Materials Required : Sodium chloride : 0.25 g ; 5%
w/v silver nitrate in DW (+ 2-3) drops of conc. HNO3 ; dilute nitric
acid (6 N) ; asbestos fibre.
Theory : The following reaction forms
the basis for the calculation of the theoretical amount of silver nitrate solution required as well as
the purity of the given sample of NaCl. Thus, we have :
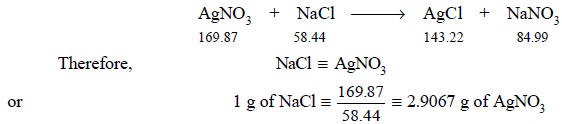
As 0.2570 g of NaCl has been used (from experimental
data); therefore, the exact amount of AgNO3 required would be :

Hence, the amount of AgNO3 solution required
theoretically would be 0.7470/0.05 = 14.94 ml.
From above, the percentage purity of the given sample of
NaCl may be found as shown below :
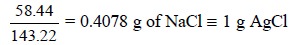
The weight of AgCl is found to be 0.6288 g
experimentally, or 0.4078 is the ‘gravimetric factor’.
Consequently, the percentage purity of the sample is
determined by the formula :
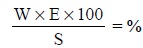
where, W = Wt. of the product of a chemical reaction with the substance
under determination,
E = Gravimetric Factor, and
S = Wt. of the sample.
By incorporating the data given above, the amount of
sodium chloride present in 100 g of the sample i.e., the percentage purity of NaCl in the given sample may be
calculated as follows :
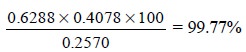
Procedure : Weigh accurately between 0.20
to 0.30 g of sodium chloride and dissolve in 100 ml of DW. Add to it 1 ml of dilute nitric acid gradually with constant
stirring. Check and confirm that the resulting solution is acidic with the help
of blue litmus paper. Measure out 5.0 ml in excess of the amount of silver
nitrate solution calculated on theoretical basis to precipitate all the
available chlorine as silver chloride. The requisite quantity of silver nitrate
solution must be added in small lots at intervals with constant stirring with a
glass rod. Cover the beaker with a watch-glass and boil the contents very
gently with occasional stirring (to avoid bumping of the liquid and loss of
volume). Stop heating and digest the mixture for 10 minutes so as to
agglomerate the precipitate and enhance settling thereby leaving a clear
supernatant liquid. Add 2 drops of silver nitrate solution to the hot
supernatant liquid in order to confirm whether precipitation is completed. Keep
the beaker away from direct sunlight to allow the precipitate to settle.
Take a properly prepared Gooch crucible, heat to constant
weight and fit it into the suction flask. Decant most of the supernatant liquid
first into the Gooch crucible by applying gentle suction to hasten filtration.
Wash the precipitate on the Gooch crucible at least thrice with 15 ml portions
of 0.01 N nitric acid.
Test the above filtrate to be free of AgNO3.
Finally wash the precipitate twice with 5 ml portion of DW to get rid of most
of the HNO3 previously retained by the precipitate from the former
wash solution. Now, apply vigorous suction to drain out the liquid from the
precipitate to the maximum extent. Dry the crucible to a constant weight
between 110-120°C in an electric oven until two concurrent weighings are
achieved. Thus, the weight of the crucible (tare) must be deducted from the
weight of the crucible plus the precipitate to arrive at the weight of silver
chloride duly obtained from the sample.
Precautions :
1.
The solution of the substance is usually acidified with
HNO3 to check the precipitation of other substances insoluble in
water but soluble in HNO3 e.g.,
CO32–, O2– and PO43–.
Besides HNO3 also helps to coagulate any colloidal AgCl,
2.
The excess of HNO3 must be avoided to cause
solvolysis of silver halides,
3.
Heating should be affected only after the addition of
AgNO3, otherwise Cl2 may be liberated and lost. Thus, we
have :
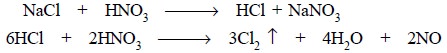
4.
The precipitation should preferably be carried out in the
absence of strong light because AgCl undergoes decomposition in sunlight with
loss of Cl2,
5.
Washing of the precipitate (AgCl) with 0.01 N HNO3
is always recommended to prevent loss of AgCl by virtue of its return to
colloidal condition (peptization) and to get rid of the soluble salts, namely :
AgNO and NaNO3, and
6.
AgCl is significantly volatile on ignition, hence it must
always be dried at a comparatively lower temperature.
2. Potassium Alum, KAl(SO4)2, 12H2O
Theory : The percentage of Al in
potassium alum can be determined volumetrically by complexometric titration.
However, gravimetric procedure provides a fairly reliable
and useful alternative method of analysis for
Al which may be accomplished by :
(a)
precipitation from a solution of the aluminium salt by the addition of NH4OH
in the presence of NH4Cl, and
(b)
complexation from a solution of the aluminium salt with 8-hydroxyquinoline
(oxine) either from an ammoniacal solution or from acetic acid-acetate buffer.
In the first method, the following reaction takes place :
Al3+ + 3OH–
→ Al(OH)3 ↓
The gelatinous white precipitate of Al(OH)3 is
duly filtered, washed with dilute NH4NO3 solution,
transformed to the corresponding oxide and finally weighed as Al2O3
.
Disadvantages : There are a number of serious
disadvantages of this method, namely :
(i) excess of
NH4OH may directly affect the solubility of Al(OH)3,
(ii)
coprecipitation of metal hydroxides that are usually soluble in NH4OH,
(iii) heated
oxide (Al2O3) is hygroscopic in nature, and
(iv) hydroxides
may not undergo complete thermal decomposition.
Due to the above short-comings, the second method is
usually preferred which shall be discussed below :
Equation :
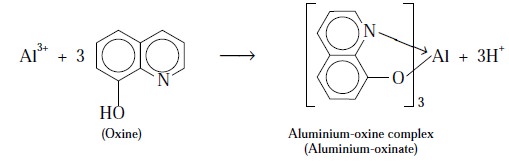
The resulting precipitate of aluminium-oxine complex is
crystalline in nature and hence can be filtered conveniently, washed with water
and finally dried at 130-150°C to constant weight.
Disadvantages : There are two disadvantages of the metal-oxine-complex method, namely :
(i)
aluminium-oxinate is prone to adsorb oxine, and
(ii) lack of
selectivity of oxine such that all metals except the alkaline earths (Ba, Mg,
Ca, Sr, Be) and alkali (Li, Na, K, Rb, Cs) should be totally absent.
Calculations :

Materials Required : Potassium alum : 0.3 g ; 0.1 N
hydrochloric acid : 1.0 ml ; 8-hydroxyquinoline reagent (or oxine-reagent) (25 ml of a 2% w/v solution of oxine in
2 N acetic acid) ; 2 N ammonium acetate (dissolve 15.0 g of ammonium acetate in
20.0 ml of DW, add 0.3 ml of glacial acetic acid and dilute to 100 ml with DW)
; sintered glass crucible No : 3 or 4.
Procedure : Weigh accurately about 0.3 g
of potassium alum in a 400-ml beaker, dissolve it in 150 ml of DW containing 1.0 ml of 0.1 N HCl and warm the contents of the
beaker to about 60°C. Add the requisite quantity of the oxine reagent and then
add a 2 N solution of ammonium acetate gradually from a pipette till
precipitation just commences. Add a further portion (50 ml) of ammonium acetate
solution with vigorous stirring. Allow the contents of the beaker to stand for
60 minutes with frequent stirring. Filter the precipitate through No : 3 or 4
sintered glass crucible that has been previously dried to a constant weight at
130—150°C. Wash the precipitate throughly with cold DW and dry at 130 to 150°C
to constant weight. Each gram of aluminium oxinate is equivalent to 0.05873 g
of Al.
3. Cognate Assays
A good deal of pharmaceutical substances are officially
assayed gravimetrically as appears in Table 10.2.
Related Topics