Chapter: Pharmaceutical Drug Analysis: Gravimetric Analysis
Substances Assayed After Conversion - Gravimetric Analysis
SUBSTANCES ASSAYED AFTER CONVERSION
There are certain pharmaceutical substances that can be
assayed gravimetrically after their suitable conversion to free acid, or free
base, or free compound or corresponding derivatives (or substitution products).
All these typical cases shall be discussed briefly with their appropriate
examples in the following sections.
1. Substances Assayed after Conversion to Free Acid
A few official pharmaceutical substances may be assayed
gravimetrically by affecting separation, purification, and weighing an organic
medicinal compound without causing any permanent change in composition. It is
an usual practice that before extraction of the organic medicinal compound, the
sample of the crushed tablets is carefully washed with petroleum benzene to get
rid of undesirable components, for instance : lubricants and binders that would
be extracted along with the organic medicinal compound by such solvents as
ether or chloroform which is employed subsequently.
In case, the organic medicinal compound is acidic in
nature e.g., amobarbital in sodium
amobarbital tablets, it is first and foremost extracted with an aqueous
solution of an acid or base to cause separation from the neutral substance
which might be present. The resulting aqueous solution of the salt of the
respective organic medicinal compound is subsequently made acidic and the
liberated organic acid (amobarbital) is finally extracted with ether or
chloroform.
Interestingly, in a situation where either magnesium
stearate or stearic acid forms a component in the formulation, the organic
medicinal compound which is acidic (amobarbital) cannot be extracted with NaOH
solution for obvious reason that sodium stearate shall also be extracted along
with the salt of the organic acid. Therefore, instead a saturated solution of
Ba(OH)2 is employed thereby the insoluble precipitate of barium
stearate may be discarded by filtration.
1.1. Phenobarbitone Sodium
Materials Required : Phenobarbitone sodium : 0.5 g
; hydrochloric acid (2 M) : (dissolve 17.0 ml (~ 11.5 N) in 100 ml DW) : 5.0 ml ; ether : 13.5
ml ; absolute ethanol : 2.0 ml.
![]()
Procedure : Weigh accurately 0.5 g
phenobarbitone sodium and dissolve in 15 ml of DW. Add to it 5 ml of 2 M hydrochloric acid and
extract with 50 ml of ether and then with successive 25 ml quantities of ether
until complete extraction is affected. Wash the combined extracts with two 5 ml
quantities of DW and wash the combined aqueous extracts with 10 ml quantities
of ether. Add the ether to the main ethereal extract, evaporate to low bulk,
add 2 ml of absolute ethanol, evaporate to dryness and dry the residue to
constant weight at 105°C. Each g of residue is equivalent to C12H11N2NaO3.
Calculations :
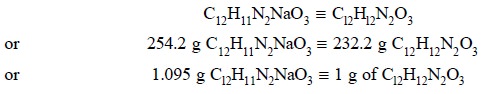
1.2. Cognate Assays
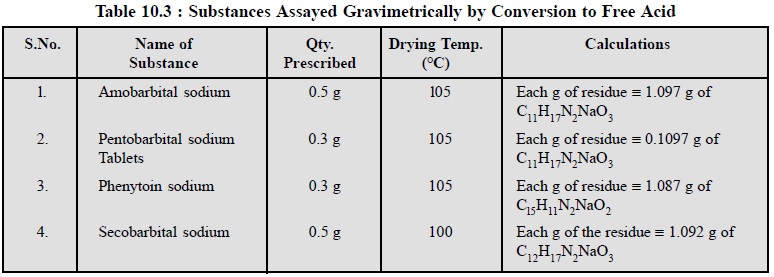
There are certain pharmaceutical substances that may be
assayed after their conversion to the respec-tive free acids as shown in Table
10.3.
2. Substances Assayed after Conversion to Free Base
In a specific instance where the organic medicinal
substance is basic in nature e.g.,
papaverine in papaverine hydrochloride, it is primarily treated with an aqueous
solution of a base and subsequently the liberated organic base is extracted
with either chloroform or ether.
A typical example is described below :
2.1. Papaverine Hydrochloride Tablets
Materials Required : Sodium hydroxide (2 M)
(dissolve 8.0 g of NaOH pellets in 100 ml of CO2 free DW : 50 ml ; chloroform : 100 ml ; absolute ethanol : 5 ml.
Calculations :
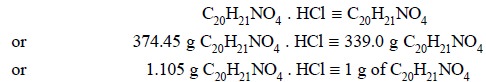
Procedure : Weigh 20 tablets and crush
them in a pestle mortar and find out the average weight of a single tablet. Accurately weigh 0.5 g
equivalent of papaverine hydrochloride and dissolve in 15 ml of DW. Add to it
15 ml of 2 M sodium hydroxide and extract with 50 ml of chloroform and then with
successive 25 ml quantities of chloroform until complete extraction is
affected. Wash the combined extracts with two 5 ml quantities of DW and wash
the combined aqueous extract with two 10 ml quantities of chloroform. Add the
chloroform to the main chloroform extract, evaporate to a small volume, add 2
ml of absolute ethanol, evaporate to dryness and dry the residue to constant
weight at 105°C.
Each g of the residue is equivalent to 1.105 g of C20H21NO4
. HCl.
2.2. Amodiaquine Hydrochloride
Materials Required : Amodiaquine hydrochloride :
0.3 g ; dilute ammonia solution (42.5 ml of strong ammonia solution to 100 ml in water) ; NO. 4 sintered glass
crucible.
Theory : Amodiaquine hydrochloride
possesses two moles of inherent water of crystallization, and hence the precentage base is
calculated with reference to the substance dried over P2O5
at a pressure not exceeding 5 mm of Hg. Usually, the assay is performed on one
portion of the sample and the drying on a separate portion altogether.
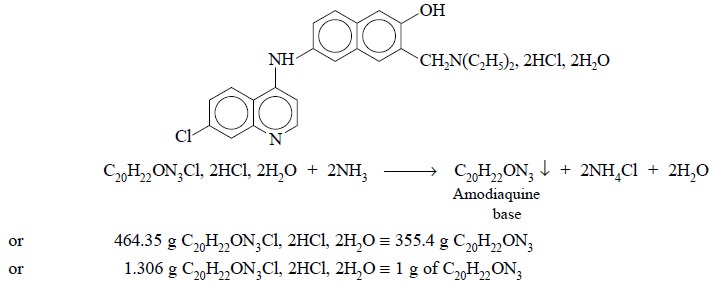
The underlying principle of the method is based upon the
precipitation of amodiaquine base that is generated as a precipitate when the
salt is decomposed in aqueous medium with dilute ammonia.
Procedure : Weigh accurately 0.3 g of
previously dried amodiaquine hydrochloride into a 100 ml beaker provided with a stirring rod and watch glass cover.
Dissolve it in 50 ml of DW and dilute ammonia solution with constant gentle
stirring until the solution is just alkaline (to litmus paper). Allow the contents
of the flask to stand for 30 minutes and then quantitatively filter through a
NO. 4 sintered glass-crucible previously dried to a constant weight at 105°C.
Wash the precipitate several times with DW, until the washings do not give a
positive test for chloride (test with standard AgNO3 Solution). Dry
the residue to a constant weight at 105°C. Each gram of residue is equivalent
to 1.306 g of C20H22ON3Cl, 2HCl, 2H2O.
2.2. Cognate Assays
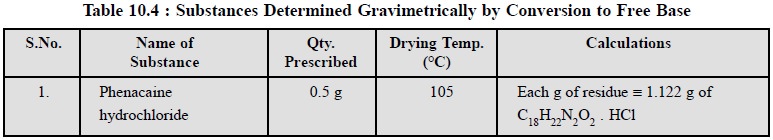
A few other pharmaceutical substances are also determined
after conversion to free bases as recorded in Table : 10.4.
3. Substances Assayed After Conversion to Free Compound
In certain specific cases either the pure pharmaceutical
substance or dosage forms are quantitatively converted to free compound. This
conversion to free compound is quantitative and hence forms the basis of
gravimetric analysis. A few typical examples belonging to this category are,
namely : progesterone suspension sterile, progesterone tablets, sodium lauryl
sulphate, mephobarbital tablets and sorbitan monooleate.
3.1. Mephobarbital Tablets
Materials Required : Mephobarbital : 300 mg ;
hexane : 100 ml ; chloroform : 150 ml ; alcohol (95% v/v) : l0 ml.
Procedure : Weigh and finely powder not
less than 20 mephobarbital tablets. Transfer an accurately weighed portion of the powder equivalent to about 300 mg of
mephobarbital to an extraction thimble. Extract with 15 ml of solvent hexane,
allow the thimble to drain, transfer to a continuous extraction apparatus
pro-vided with a tared flask, and extract the mephobarbital with chloroform for
2 hours. Evaporate the chloroform on a steam bath with the aid of a current of
air, cool, dissolve the residue in about 10 ml of alcohol, evaporate, dry the
residue at 105°C for 1 hour, cool and weigh.
The weight of the residue represents the weight Cl3H14N2O3
in the portion of the tablets taken.
4. Substances Assayed after Conversion to Derivatives or Substitution Products
In pharmaceutical drug analysis a host of organic
pharmaceutical substances are invariably converted quantitatively to their
corresponding derivatives by virtue of interactions with certain functional
entities, namely : aldehyde, ketone, amino, carboxyl, phenolic, hydroxyl etc.
However, in some cases it may be feasible to obtain uniform substitution
products of organic pharmaceutical substances quantitatively, for instance :
tetraido derivative of phenolphthalein is obtained from the phenolphthalein
tablets. It is important to mention here that the number of organic pharmaceutical
substances which may be analysed by this method is limited because of two vital reasons, they are :
(a) the
reversible nature of reactions, and
(b) the
formation of products of side reactions simultaneously.
4.1. Benzylpenicillin(Syn : Benzylpenicillin
Sodium or Potassium Salt)
Materials Required : Benzylpenicillin sodium (say)
: 0.12 g ; amyl acetate (previously saturated with 1-ethylpiperidinium benzylpencillin at room temperature, cooled in
ice and filtered) : 5.0 ml ; phosphoric acid (20% v/v) : 0.5 ml ; anhydrous
sodium sulphate (freshly ignited and powdered) : 0.5 g ; dry acetone
(previously saturated with 1-ethylpiperidinium benzylpenicillin at room
temperature cooled in ice and filtered) : 3.0 ml ; 1-ethylpiperidine amyl
acetate solution (prepared from l-ethyl piperidine, 1 .0 ml, and amyl acetate,
8.0 ml, saturated at room temperature with 1-ethylpiperidinium
benzylpenicillin, cooled in ice and filtered) : 1.5 ml ; dry acetone in amyl
acetate (1 : 1) previously saturated with 1-ethylpiperidinium benzylpenicillin
: 2.0 ml ; solvent ether : 4.0 ml.
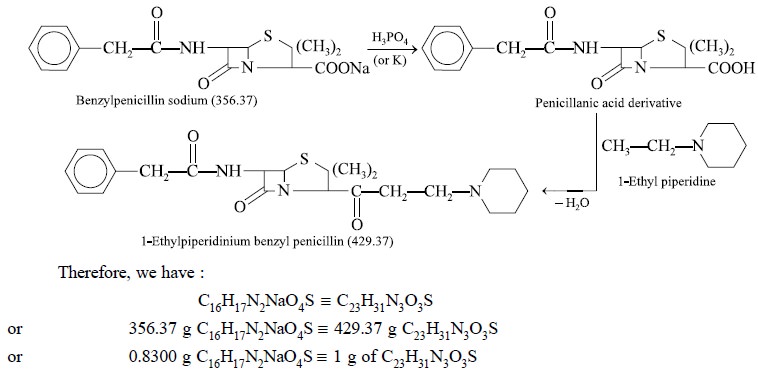
Theory : Benzylpenicillin (sodium or
potassium salt) may be assayed gravimetrically by quantitative conversion to the 1-ethylpiperidinium
benzylpenicillin derivative. The ultimate precipitation is caused by l-ethyl
piperidine after the respective sodium or potassium salt of benzylpencillin has
been duly converted with phosphoric acid to the corresponding penicillanic acid
(i.e. parent acid) and the latter
finally extracted with amyl alcohol. The reactions may be expressed as follows
:
Procedure : Weigh accurately 0.12 g of
benzyl penicillin sodium, dissolve in 5 ml of ice-cold DW in a flask and cool in an ice-bath. Add to it 5.0 ml of amyl
acetate followed by 0.5 ml of ice-cold H3PO4, stopper,
shake the contents immediately for 15 seconds, and centrifuge for 30 seconds.
Remove the aqueous layer as completely as possible with the help of a pipette.
Add 0.5 g anhydrous Na2SO4, stir the contents vigorously
and cool in an ice-bath for 5 minutes. Centrifuge for about 30 seconds and
again cool in ice-bath for 5 minutes. Pipette 3.0 ml of the supernatant liquid
into a tared centrifuge tube. Add to it 3.0 ml of ice-cold acetone and 1.5 ml
of 1-ethylpiperidine amyl acetate solution, stir, stopper the tube and cool in
ice-bath for 2 hours. Now, centrifuge for 1 minute, break the surface with the
help of a pointed glass rod, so that all crystalline particles are covered by
liquid, and again centrifuge for 1 minute. Decant off the supernatant liquid,
wash the precipitate with 2 ml of ice-cold dry acetone in amyl acetate (1 : 1)
and again centrifuge for 1.5 minutes. Decant the supernatant liquid, wash twice
with 2.0 ml portion of solvent ether, centrifuging for 1.5 minutes and
decanting each time. Dry to constant weight under vacuum at room temperature.
Each gram of residue is equivalent to 0.8300 g of C16H17N2NaO4S.
4.2. Cholesterol
Materials Required : Cholesterol : 0.1 g ; ethanol
(90% v/v) : 12.0 ml ; digitonin solution (0.5% w/v in 90% v/v ethanol) : 40.0
ml ; ethanol (90% v/v) : 100 ml ; acetone ; carbon tetrachloride.
Theory : The assay of cholesterol is
solely based on the fact that practically all 3 β-hydroxysterols e.g., cholesterol, readily produces an insoluble molecular addition
complex with pure digitonin (1 : 1)—a steroidal saponin isolated from either Digitalis purpurea or Digitalis lanata. The complex thus
obtained is crystalline in nature, fairly stable and possesses very low
solubilities.
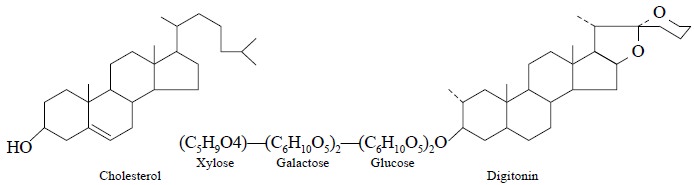
The complexation of cholesterol and digitonin may be
expressed as follows :
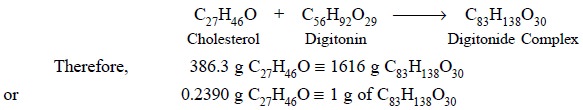
Procedure : Weigh accurately about 0.1 g
of cholesterol into a 100 ml flask and dissolve it in 12.0 ml ethanol. Insert the stopper and allow
to stand at room temperature (25 ± 2°C) for 12 hours, filter through a Gooch
crucible, and wash with 5.0 ml of ethanol. Mix the washings to the filtrate and
add to it 40.0 ml solution of digitonin and make it warm to 60°C to ensure that
the complexation is almost complete. Filter the precipitate of the resulting
complex through a prepared Gooch crucible, previously dried to constant weight
at 105°C. Wash the precipitate with ethanol followed by acetone and carbon
tetrachloride, allow to drain as completely as possible, and dry to a constant
weight at 105°C. Each g of the residue is equivalent to 0.2390 g of
cholesterol.
Note : All solutions must be
ice-cold.
4.3. Thiamine Hydrochloride
Materials Required : Thiamine hydrochloride : 0.5 g
; hydrochloric acid ( ~ 11.5
N) : 2.0 ml ; ![]() silicotungstic acid solution
(10% w/v in water) : 4.0 ml ; NO : 4-sintered glass-crucible ; dilute
hydrochloric acid (1 part HCl + 19 parts H2O) : 50 ml.
silicotungstic acid solution
(10% w/v in water) : 4.0 ml ; NO : 4-sintered glass-crucible ; dilute
hydrochloric acid (1 part HCl + 19 parts H2O) : 50 ml.
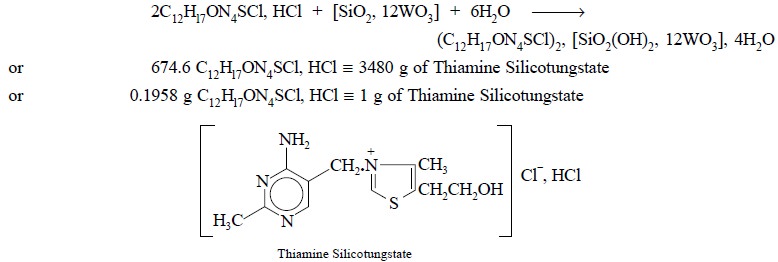
Theory : The gravimetric assay of
thiamine hydrochloride is based upon the precipitation of it as thiamine silicotungstate with
silicotungstic acid in a slightly acidic medium. It has been observed that the
precipitating reagent is a complex silicate SiO2, 12 WO2,
n H2O having somewhat
variable composition with regard to the degree of hydration. For a reasonably
precise and accurate determination the precipitating reagent must contain <|
1.85% SiO2 and <| 85% WO3.
Interestingly, the thiamine silicotungstate complex possesses more or less a
constant composition.
The precipitation of insoluble thiamine silicotungstate
may be designated by the following reaction :
Procedure : Weigh accurately 0.05 g of
thiamine hydrochloride, previously dried at 105°C, and dissolve it in 50 ml DW in a 250 ml beaker having a stirring rod
and watch glass cover. Add to it 2.0 ml of hydrochloric acid, heat to boiling
and then add 4.0 ml of silicotungstic acid solution as rapidly as possible.
Now, boil the solution gently for 2 minutes and quickly filter through a NO. 4
sintered-glass crucible, previously dried to a constant weight at 105°C. Wash
the residue with a boiling mixture of HCl and H2O (1 : 19) about 40
ml, then with DW 10.0 ml and ultimately with two portions of 5 ml each of
acetone. Finally dry the residue to constant weight at 105°C. Each g of
thiamine silicotungstate residue is equivalent to 0.1938 g of C12Hl7ON4SCl,
HCl.
Precautions :
(a) An excess
of HCl is a must so as to produce a readily filterable precipitate,
(b) In case the
sample is pure enough, the rate of addition of silicotungstic acid has little
influence on the result, but on the contrary if the sample has significant
impurity it may afford poor results,
(c) To achieve
complete complexation boiling must be done for more than 2 minutes, otherwise
it would yield low results, and
(d) A 50-ml
wash-liquid is quite ideal, further washings (volume) may offer poor results.
4.4. Histamine Acid Phosphate (C5H9N3,
2H3PO4)
Materials Required : Histamine : 0.15 g ;
nitranilic acid solution (3.5% w/v in 95% ethanol) : 10.0 ml ; ethanol (95%) :
30.0 ml ; sintered-glass crucible (NO : 3) ; ether : 10.0 ml.
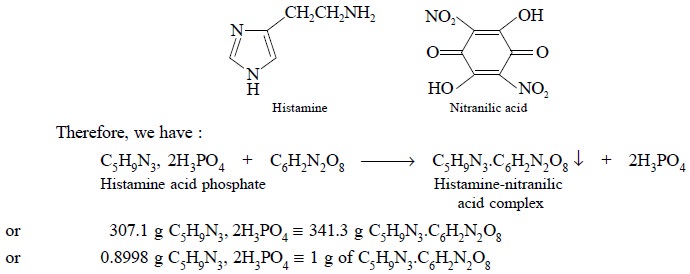
Theory : The gravimetric assay of
histamine acid phosphate is based upon the formation of insoluble histamine-nitranilic acid complex as
depicted in the following equation :
Procedure : Weigh accurately about 0.15 g
of histamine acid phosphate into a 250 ml beaker provided with a stirring rod and watch glass cover. Add to it 10.0
ml of DW to dissolve the sample. Now, add 10.0 ml of nitranilic acid solution,
stir and allow to stand for 15 ininutes. Pour in 10.0 ml of ethanol, keep it in
an ice-bath for 3 hours and filter through a No. 3 sintered-glass crucible,
previously dried to a constant weight at 130°C. Transfer the precipitate
quantitatively and wash it thoroughly with four quantities each of 5.0 ml of
ethanol and ultimately with 10.0 ml of ether. Dry to constant weight at 130°C.
Simultaneously, determine the loss in weight on drying a separate portion of
the sample at 105°C. Each gram of the histamine-nitranilic acid complex is
equivalent to 0.8998 g of C5H9N3, 2 H3PO4.
4.5. Proguanil Hydrochloride
Materials Required : Proguanil hydrochloride : 0.6
g ; ammoniacal cupric chloride solution (dissolve 22.5 g of copper (II) chloride in 200 ml of DW and mix with 100 ml
of 13.5 M ammonia) ; NO. 4 sintered-glass crucible ; mixture of dilute solution
of ammonia and DW (1 : 5).
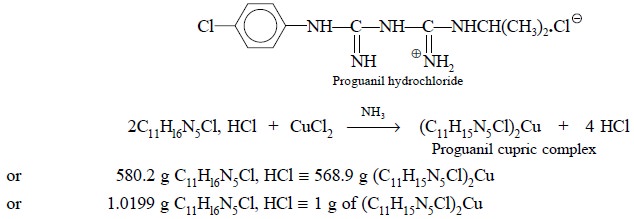
Theory : Gravimetric analysis of
proguanil hydrochloride involves the precipitation of the proguanil-cupric
complex that results on the addition of ammoniacal cupric chloride solution to
a solution of proguanil hydrochloride. The reaction can be expressed by the
following equation :
Procedure : Weigh accurately 0.6 g of
proguanil hydrochloride into a 250 ml beaker fitted with a stirring rod and watch-glass cover. Add to it 50.0 ml of DW and
heat gently to dissolve the sample. Chill the solution below 10°C in an
ice-bath and then add ammoniacal-cupric-chloride solution with continuous
stirring till the resulting solution attains a permanent deep-colour. Allow the
solution to stand for 90 minutes to complete the complexation and then filter
through a No. 4 sintered glass crucible previously dried to constant weight at 130°C.
Transfer the precipitate quantitatively into the crucible, wash first with a
mixture of dilute solution of ammonia and DW (1 : 5) adequately followed by
cold water until the washings are practically colourless thereby showing the
complete absence of soluble copper salts. Dry the precipitate to a constant
weight at 130°C. Simultaneously, find out the loss in weight on drying with a
separate portion of the sample at 105°C and incorporate this in the
calculation. Each gram of proguanil-cupric-complex is equivalent to 1.0199 g of
C11Hl6N5Cl, HCl.
4.6. Benzethonium Chloride
Theory : In general, quaternary
nitrogen containing compounds like—choline chloride, acetylpyridinium chloride, benzethonium chloride, and
bethanechol chloride readily form insoluble salts quantitatively with
tetraphenyl boron and this puts forward the basis for the gravimetric assay of
the above cited pharmaceutical substances.
The various reactions involved may be summarized and
expressed as follows :
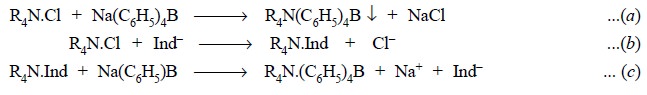
Eq. (a) shows
that the quaternary salt gets quantitatively precipitated by sodium tetraphenyl
boron as the complexing agent. Eq. (b)
depicts that quaternary compounds shall readily react with certain anionic dye,
such as bromophenol blue, to yield a blue, chloroform-soluble complex.
Eq. (c) finally
illustrates that the blue-coloured complex shall react quantitatively with
sodium tetraphenyl boron to give an insoluble compound.
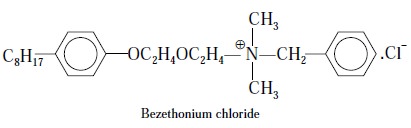
Therefore, we have :
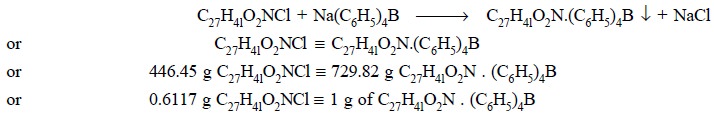
Materials Required : Benzethonium chloride : 0.15 g
; Chloroform : 50 ml ; bromophenol blue solution
(Dissolve with heating 0.2 g of bromophenol blue in 3 ml of 0.1 M NaOH and 10
ml of ethanol (96%). Allow to cool and dilute to 100 ml with ethanol 96%] : 50
ml ; sodium tetraphenyl borate solution (1% w/v in chloroform) : 50 ml ;
sintered-glass crucible No : 4.
Procedure : Weigh accurately about 0.15 g
of benzethonium chloride sample into a 250-ml beaker placed on a magnetic-stirrer and watch-glass cover. Add to it 25
ml of chloroform and warm gently to dissolve. Cool to ambient temperature and
add suffcient bromophenol blue solution gradually till the solution yields a
blue Chloroform-soluble complex. Now, add sodium tetraphenyl borate solution in
small lots at intervals with constant stirring until the complete precipitation
of insoluble benzethonium tetraphenyl borate complex takes place. Allow the
solution to stand for 60 minutes to complete the complexation and subsequently
filter through a No. 4 sintered-glass crucible previously dried to constant
weight at 130°C. Transfer the precipitate quantitatively into the crucible and
wash the precipitate with cold chloroform. Dry the precipitate to a constant
weight at 110°C. Each gram of benzethonium tetraphenyl borate complex is
equivalent to 0.6117 g of C27H4lO2NCl.
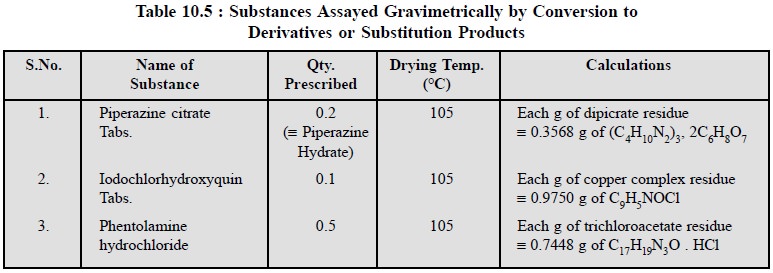
4.7. Cognate Assays
Quite a few official pharmaceutical substances and their
respective dosage forms can be assayed gravimetrically after conversion to
their corresponding derivatives or substitution products. Table 10.5 records
some examples from official compendia.
Related Topics