Chapter: Modern Pharmacology with Clinical Applications: General Organization and Functions of the Nervous System
Steps in Neurochemical Transmission
STEPS IN
NEUROCHEMICAL TRANSMISSION
Regardless of the type of
neuron under consideration, the fundamental steps in chemical transmission are
the same. Each of these steps is a potential site for phar-macological
intervention in the normal transmission process:
1. Synthesis of the
transmitter
2. Storage of the transmitter
3. Release of the transmitter
by a nerve action potential
4. Interaction of the
released transmitter with receptors on the effector cell membrane and the
associated change in the effector cell
5. Rapid removal of the
transmitter from the vicinity of the receptors
6. Recovery of the effector cell to the state that preceded transmitter action
Synthesis, Storage, Release, and Removal of Acetylcholine
The processes involved in
neurochemical transmission in a cholinergic neuron are shown in Figure 9.2. The
ini-tial substrates for the synthesis of acetylcholine are glu-cose and choline. Glucose
enters the neuron by means of
facilitated transport. There is some disagreement as to whether choline enters
cells by active or facilitated transport. Pyruvate derived from glucose is
transported into mitochondria and converted to acetylcoenzyme A (acetyl-CoA).
The acetyl-CoA is transported back into the cytosol. With the aid of the enzyme
choline acetyl-transferase, acetylcholine is synthesized from acetyl-CoA and
choline. The acetylcholine is then transported into and stored within the
storage vesicles by as yet un-known mechanisms.
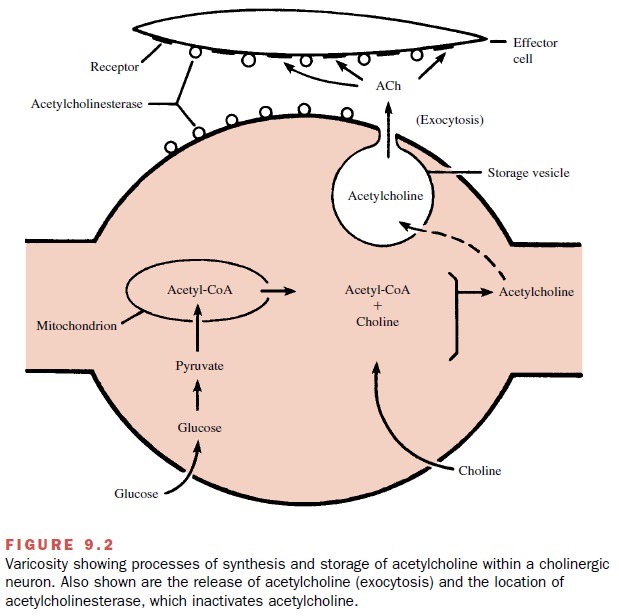
Conduction of an action
potential through the ter-minal branches of an axon causes depolarization of
the varicosity membrane, resulting in the release of trans-mitter molecules via
exocytosis. Once in the junctional
extracellular space (biophase), acetylcholine interacts with cholinoreceptors.
A key factor in the process
of exocytosis is the entry of extracellular calcium ions during the
depolarization.
Modification of extracellular
calcium concentration or of calcium entry therefore can markedly affect
neuro-transmission.
The interactions between
transmitters and their receptors are readily reversible, and the number of
transmitter–receptor complexes formed is a direct func-tion of the amount of
transmitter in the biophase. The length of time that intact molecules of
acetylcholine re-main in the biophase is short because acetylcholinesterase, an enzyme that rapidly hydrolyzes
acetylcholine, is highly concentrated on the outer surfaces of both the
prejunc-tional (neuronal) and postjunctional (effector cell) mem-branes. A
rapid hydrolysis of acetylcholine by the enzyme results in a lowering of the
concentration of free trans-mitter and a rapid dissociation of the transmitter
from its receptors; little or no acetylcholine escapes into the
circu-lation.Any acetylcholine that does reach the circulation is immediately
inactivated by plasma esterases.
The rapid removal of
transmitter is essential to the exquisite control of neurotransmission. As a
conse-quence of rapid removal, the magnitude and duration of effect produced by
acetylcholine are directly related to the frequency of transmitter release,
that is, to the fre-quency of action potentials generated in the neuron.
Synthesis, Storage, Release, and Removal of Norepinephrine
Transmission in noradrenergic
neurons is somewhat more complex, particularly in regard to the mechanisms by
which the transmitter is removed from the biophase subsequent to its release.
Noradrenergic transmission is represented diagrammatically in Figure 9.3.
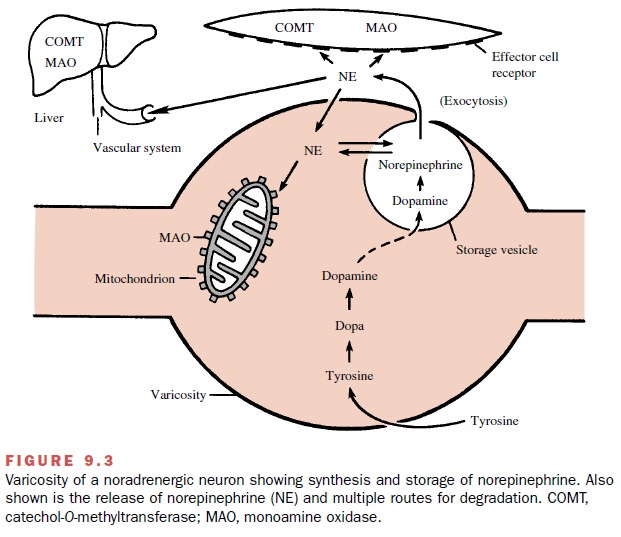
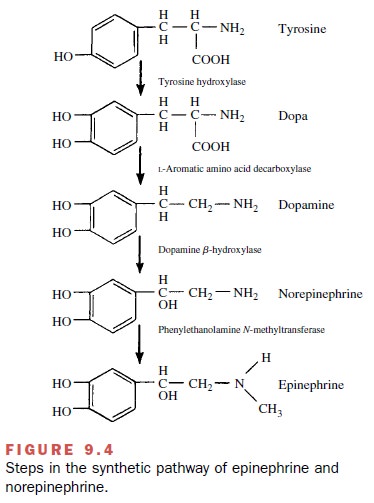
Synthesis of norepinephrine
begins with the amino acid tyrosine,
which enters the neuron by active trans-port, perhaps facilitated by a
permease. In the neuronal cytosol, tyrosine is converted by the enzyme tyrosine hy-droxylase to dihydroxyphenylalanine (dopa), which is converted to dopamine by
the enzyme aromatic L–amino acid decarboxylase, sometimes termed dopa-decarboxylase. The dopamine is actively transported into storage vesicles, where it is
converted to norepi-nephrine (the transmitter) by dopamine -hydroxylase, an enzyme within the storage vesicle.
In noradrenergic neurons, the
end product is norepi-nephrine. In the adrenal medulla, the synthesis is
carried one step further by the enzyme phenylethanolamine
N-methyltransferase, which converts
norepinephrine to epinephrine. The human adrenal medulla contains ap-proximately
four times as much epinephrine as norepi-nephrine. The absence of this enzyme
in noradrenergic neurons accounts for the absence of significant amounts of
epinephrine in noradrenergic neurons. The structures of these compounds are
shown in Figure 9.4.
Since the enzyme that
converts dopamine to norepi-nephrine (dopamine -hydroxylase) is located only
within the vesicles, the transport of dopamine into the vesicle is an essential
step in the synthesis of norepi-nephrine. This same transport system is
essential for the storage of norepinephrine. There is a tendency for
nor-epinephrine to leak from the vesicles into the cytosol. If norepinephrine
remains in the cytosol, much of it will be destroyed by a mitochondrial enzyme,
monoamine oxidase (MAO). However, most of the norepinephrine that leaks out of the vesicle is
rapidly returned to the storage vesicles by the same transport system that
car-ries dopamine into the storage vesicles. It is important for a proper
understanding of drug action to remember that this single transport system,
called vesicular trans-port, is an essential element of both synthesis and
storage of norepinephrine.
Like the cholinergic
transmitter, the noradrenergic transmitter is released by action potentials
through ex-ocytosis, the contents of entire vesicles being emptied into the
biophase (synaptic or junctional region). Similarly, the formation of
transmitter–receptor com-plexes is a direct function of the concentration of
trans-mitter in the biophase and is readily reversible. In this instance, the
receptors are adrenoceptors.
Three processes contribute to
the removal of nor-epinephrine from the biophase:
· Transport back into the
noradrenergic neuron (reup-take),
followed by either vesicular storage or by en-zymatic inactivation by
mitochondrial MAO. The transport of norepinephrine into the neurons is a
sodium-facilitated process similar to that for choline transport.
· Diffusion from the synapse
into the circulation and ultimate enzymatic destruction in the liver and renal
excretion.
· Active transport of the
released transmitter into ef-fector cells (extraneuronal
uptake) followed by enzy-matic inactivation by catechol-O-methyltransferase.
The neuronal transport system
is the most impor-tant mechanism for removing norepinephrine. Any
nor-epinephrine or epinephrine in the circulation will equil-ibrate with the
junctional extracellular fluid and thus become accessible both to the receptors
and to neu-ronal transport. Thus, neuronal transport is also an im-portant
mechanism for limiting the effect and duration of action of norepinephrine or
epinephrine, whether these are released from the adrenal medulla or are
ad-ministered as drugs. Neuronal uptake
is primarily a mechanism for removing
norepinephrine rather than conserving it. Under most circumstances,
synthesis of new norepinephrine is
quite capable of keeping up with the needs of transmission, even in the
complete absence of neuronal reuptake.
It is important to make a
clear distinction between neuronal and vesicular transport. Neuronal transport occurs from the
junctional extracellular fluid (biophase) across the cell membrane of the
neuron and into the neuronal cytosol. Vesicular
transport is from the neu-ronal cytosol across the membrane of the vesicle
and into the vesicle. Although these two systems readily transport both
norepinephrine and epinephrine, certain drugs will selectively inhibit one or
the other transport system.
The second most important
mechanism for remov-ing norepinephrine from the synapse is the escape of
neuronally released norepinephrine into the general circulation and its
metabolism in the liver. The liver has two enzymes that perform this function: catechol-O-methyltransferase (COMT)
and MAO.
COMT is a specific enzyme,
accepting only catechols as substrates. A catechol is a substance with two
adja- cent hydroxyl groups on an unsaturated six-member ring. The end result of
the action of COMT is the O-methylation
of the meta-hydroxyl group on the catechol nucleus. Figure 9.5 illustrates the
action of COMT on norepinephrine or epinephrine. This reaction reduces the
biological activity of norepinephrine or epinephrine at least 100-fold.
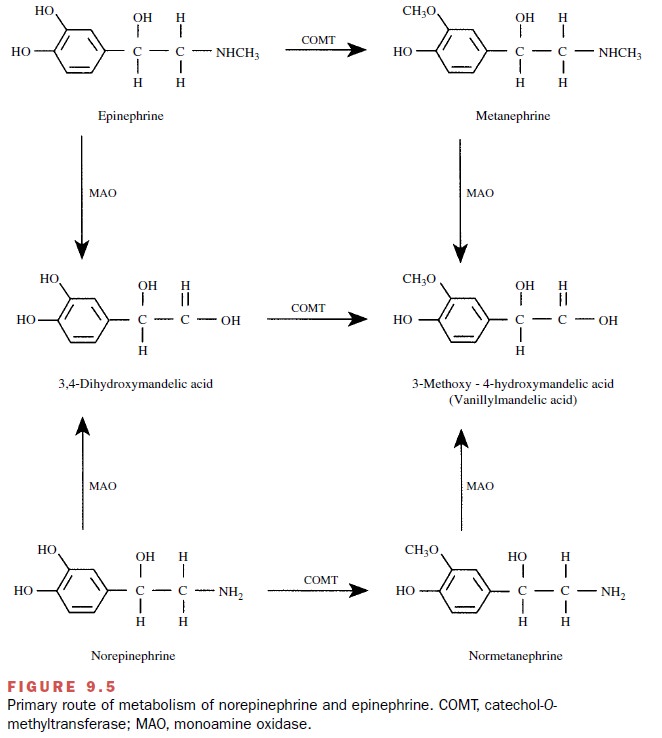
MAO is a much less discriminating enzyme in that it will catalyze the removal of an amine group from a va-riety of substrates. The action of MAO on norepineph-rine and epinephrine also is indicated in Figure 9.5. The list of its substrates is very large, including endogenous substances (norepinephrine, epinephrine, dopamine, tyramine, 5-hydroxy-tryptamine) and many drugs that are amines. At least in the brain, two separate forms of MAO have been described: MAO type A and MAO type B. The two types are differentiated on the basis of substrate and inhibitor specificity.
Although either COMT or MAO
may act first on circulating norepinephrine or epinephrine, COMT is the more
rapidly acting enzyme, and therefore more molecules are O-methylated and then deaminated than the reverse. Some norepinephrine
and epinephrine ap-pear unchanged in the urine. The larger portion, how-ever,
is metabolized and the products of metabolism ex-creted in the urine, often as
conjugates.
Measurements of
norepinephrine, epinephrine, and their metabolites in the urine constitute
valuable diag-nostic aids, particularly in the detection of tumors that
synthesize and secrete norepinephrine and epinephrine (e.g., pheochromocytoma).
Catecholamines can be
transported into effector cells (extraneuronal
uptake). These cells generally con-tain both COMT and MAO. The combined
processes of extraneuronal uptake and O-methylation
are believed to be a minor but functionally significant, site of irre-versible
loss of catecholamines. The precise role of ex-traneuronal MAO in transmitter inactivation
remains unknown.
Related Topics