Chapter: Basic & Clinical Pharmacology : Introduction to Toxicology: Occupational & Environmental
Solvents - Specific Chemicals
SOLVENTS
Halogenated Aliphatic Hydrocarbons
These agents once
found wide use as industrial solvents, degreas-ing agents, and cleaning agents.
The substances include carbon tetrachloride, chloroform, trichloroethylene,
tetrachloroethylene (perchloroethylene), and 1,1,1-trichloroethane (methyl
chloro-form). However, because of the likelihood that halogenated ali-phatic
hydrocarbons are carcinogenic to humans, carbon tetrachloride and
trichloroethylene have largely been removed from the workplace.
Perchloroethylene and trichloroethane are still in use for dry cleaning and
solvent degreasing, but it is likely that their use will be very limited in the
future. Dry cleaning as anoccupation is listed as a class 2B
carcinogenic activity by the International Agency for Research Against Cancer
(IARC). Fluorinated aliphatics such as the freons and closely related com-pounds
have also been used in the workplace and in consumer goods, but because of the
severe environmental damage they cause, their use has been limited or
eliminated by international treaty agreements. The common halogenated aliphatic
solvents also create serious problems as persistent water pollutants. They are
widely found in both groundwater and drinking water as a result of poor
disposal practices.
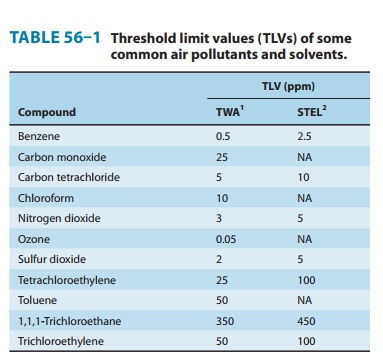
See
Table 56–1 for recommended TLVs.
A. Mechanism of Action and Clinical Effects
In laboratory animals,
the halogenated hydrocarbons cause central nervous system depression, liver
injury, kidney injury, and some degree of cardiotoxicity. Several are also
carcinogenic in animals and are considered probable carcinogens in humans.
Trichloroethylene and tetrachloroethylene are listed as “reasonably anticipated
to be a human carcinogen” by the US National Toxicology Program, and as class
2A probable human carcinogens by IARC. These sub-stances are depressants of the
central nervous system in humans; chloroform is the most potent. Chronic
exposure to tetrachloro-ethylene and possibly 1,1,1-trichloroethane can cause
impaired memory and peripheral neuropathy. Hepatotoxicity is also a com-mon
toxic effect that can occur in humans after acute or chronic exposures; carbon
tetrachloride is the most potent of the series. Nephrotoxicity can occur in
humans exposed to carbon tetrachlo-ride, chloroform, and trichloroethylene.
With chloroform, carbon tetrachloride, trichloroethylene, and
tetrachloroethylene, carcino-genicity has been observed in lifetime exposure
studies performed in rats and mice and in some human epidemiologic studies.
Reviews of the epidemiologic literature on the occupational expo-sure of
workers to various halogenated aliphatic hydrocarbon sol-vents including trichloroethylene
and tetrachloroethylene have found significant associations between exposure to
the agent and renal, prostate, and testicular cancer. Other cancers have been
found to be increased but their incidence has not reached statisti-cal
significance.
B. Treatment
There is no specific
treatment for acute intoxication resulting from exposure to halogenated
hydrocarbons. Management depends on the organ system involved.
Aromatic Hydrocarbons
Benzene is used for its solvent properties and as an intermediatein the
synthesis of other chemicals. The 2008 recommended TLVs are given in Table
56–1. Benzene remains an important compo-nent of gasoline and may be found in
premium gasolines at con-centrations as high as 2%. In cold climates such as
Alaska, benzene concentrations in gasoline may reach 5%. The PEL promulgated by
OSHA is 1 ppm in the air and a 5 ppm limit for skin exposure. The National
Institute for Occupational Safety and Health (NIOSH) and others have
recommended that the exposure limitsfor benzene be further reduced to 0.1 ppm
because excess blood cancers occur at the current PEL. The acute toxic effect
of benzene is depression of the central nervous system. Exposure to 7500 ppm
for 30 minutes can be fatal. Exposure to concentrations larger than 3000 ppm
may cause euphoria, nausea, locomotor problems, and coma; vertigo, drowsiness,
headache, and nausea may occur at concentrations ranging from 250 to 500 ppm.
No specific treat-ment exists for the acute toxic effect of benzene.
Chronic exposure to
benzene can result in very serious toxic effects, the most significant of which
is bone marrow injury. Aplastic anemia, leukopenia, pancytopenia, and
thrombocytopenia occur at higher levels of exposure, as does leukemia. Chronic
expo-sure to much lower levels has been associated with leukemia of several
types as well as lymphomas, myeloma, and myelodysplastic syndrome. Recent
studies have shown the occurrence of leukemia following exposures as low as 2
ppm-years. The pluri-potent bone marrow stem cells appear to be a target of
benzene or its metabo-lites and other stem cells may also be targets.
Epidemiologic data confirm a causal association between benzene exposure and an
increased incidence of leukemia in workers. Most organizations now classify
benzene as a known human carcinogen.
Toluene (methylbenzene) does not possess the myelotoxicproperties of
benzene, nor has it been associated with leukemia. It is, however, a central
nervous system depressant and a skin and eye irritant. It is also fetotoxic.
See Table 56–1 for the TLVs. Exposure to 800 ppm can lead to severe fatigue and
ataxia; 10,000 ppm can produce rapid loss of consciousness. Chronic effects of
long-term toluene exposure are unclear because human studies indicating
behavioral effects usually concern exposures to several solvents. In limited
occupational studies, however, metabolic interactions and modification of
toluene’s effects have not been observed in work-ers also exposed to other
solvents. Less refined grades of toluene contain benzene.
Xylene (dimethylbenzene)
has been substituted for benzene inmany solvent degreasing operations. Like
toluene, the threexylenes
do not possess the myelotoxic properties of benzene, nor have they been
associated with leukemia. Xylene is a central ner-vous system depressant and a
skin irritant. Less refined grades of xylene contain benzene. Estimated TLV-TWA
and TLV-STEL are 100 and 150 ppm, respectively.
Related Topics