Chapter: Biotechnology Applying the Genetic Revolution: Gene Therapy
Retrovirus Gene Therapy
RETROVIRUS
GENE THERAPY
Retroviruses infect many types of cells in mammals. They need dividing cells for successful
infection, and will not infect many tissues where host cell growth and division
have come to a standstill. Moreover, the genetic material of retroviruses
passes through both DNA and RNA stages. This means that introns must be removed
from any therapeutic genes before they are cloned into a retrovirus. Despite
these extra technical difficulties, a retrovirus has the distinction of
carrying the first gene in successful human gene therapy (see later
discussion).
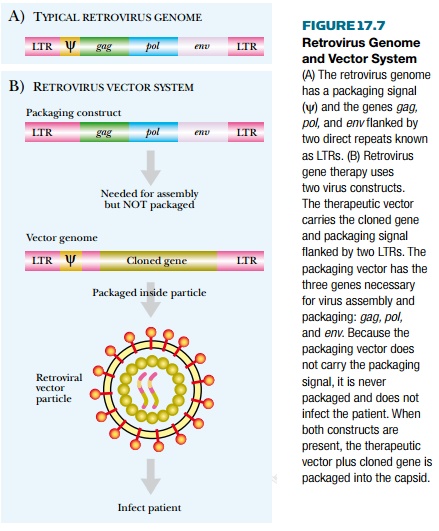
The retrovirus particle has
an inner nucleocapsid consisting of an RNA genome inside a protein shell and an
outer envelope, derived from the cytoplasmic membrane of the previous host
cell. The basic retrovirus genome consists of three genes (gag, pol, and env)
enclosed between two long terminal
repeats (LTRs), although more complex retroviruses such as HIV have extra
genes involved in regulation. The LTR sequences are needed for integration of
the DNA version of the virus genome into the host cell DNA. Between the
upstream or 5′ LTR and the gag gene is the packaging signal (Fig. 17.7), which is essential for packaging the
RNA into the virus particle.
Vectors for gene therapy have
been derived from the simpler retroviruses, especially murine leukemia virus
(MuLV). The vectors have all the retrovirus genes removed, and as a result they
are completely defective in replication. They retain only the packaging signal
and the two LTRs (Fig. 17.7) and can carry approximately 6 to 8 kb of inserted
DNA. A virus promoter in the 5′ LTR drives expression of the cloned gene.
Because the vector lacks gag, pol, and env it cannot make virus particles.
Hence these functions must be provided by a packaging construct, a defective
provirus that is integrated into the DNA of the producer cell (see Fig. 17.7).
The packaging construct lacks the packaging signal, so although it is
responsible for manufacture of virus particles, it is not packaged itself. The
virus particles generated contain only the retrovirus vector carrying the
cloned gene.
After infection of the
patient, the RNA inside the retroviral vector is reverse transcribed to give a
DNA copy. (Although the retroviral vector does not carry a copy of the reverse
transcriptase gene, a few molecules of reverse transcriptase enzyme are
packaged in retrovirus particles.) Ideally, the cloned gene, enclosed between
the two LTR sequences, is then integrated into host cell DNA. Because the
retroviral vectors are completely devoid of genes for retrovirus proteins, they
do not cause an immune response or significant inflammation. Furthermore, their
ability to integrate into host cell DNA means that the therapeutic gene will
become a permanent part of the host cell genome. In principle the retrovirus
could integrate into a harmful location, thus disrupting the function of
regulation of a host cell gene. In practice, because most DNA in animal cells
is noncoding, the chances are low, and only occasional cells would be damaged.
More serious problems are
that retroviral vectors can carry only small amounts of DNA (about 8 kb) and
cannot infect nondividing cells. However, the lentivirus family of retroviruses
(to which HIV belongs) is unusual in being able to infect some nondividing
cells. Naturally, using HIV itself is risky, but a future possibility is to
transfer this ability into other, safer retroviruses. Alternatively,
lentiviruses that infect other mammals, such as FIV (feline immunodeficiency
virus), might be used to derive vectors.
Related Topics