Chapter: Biotechnology Applying the Genetic Revolution: Gene Therapy
Aggressive Gene Therapy for Cancer
AGGRESSIVE
GENE THERAPY FOR CANCER
Although most cancers are not
inherited via the germ line, cancer is nonetheless a genetic disease. In the
case of hereditary disease we may attempt to replace the defective component,
thus preventing cell death. In contrast, when dealing with a cancer we need to
destroy the cancer cells, or at least inhibit their growth and division.
Several strategies have been used and may be classified as follows:
(a) Gene replacement
(b) Direct attack
(c) Suicide
(d) Immune provocation
Gene replacement therapy for
cancer is analogous to its use in correcting hereditary defects. The cancer is
analyzed to identify the mutant gene(s) that are responsible. The wild-type
version of the oncogene or tumor suppressor gene is then inserted into the
cancer cells. For example, the wild-type version of the p53 gene has been
delivered to p53-deficient cancer cells. The delivery method is usually via an
adenovirus vector, but sometimes liposomes have been used.
In the direct plan of attack,
a gene that helps kill cancer cells is used. For example, the TNF
gene encodes tumor necrosis factor.
This is produced by white blood cells known as tumor-infiltrating lymphocytes (TILs). These cells normally
infiltrate into tumors where they release
TNF, which is fairly effective at eradicating small cancers. To attack a large
cancer that is out of control, TNF production must be increased. First the TNF gene is cloned. Then white blood
cells are removed from the patient and cultured. Multiple copies of the TNF gene—or perhaps an improved TNF gene with enhanced activity—are
introduced into the white cells. Then the white cells are injected back into
the patient.
Although TNF is very
effective in killing cancer cells, it is also toxic to other cells. Thus high
levels of TNF are dangerous to the patient. There are two sides to this
problem. One is limiting TNF or other toxic agents to the cancer cells. The
other is getting the toxic agent to the relatively inaccessible cells on the
inside of a tumor. A variety of modifications are being tested to solve these
problems—for example, putting the TNF gene under control of an inducible
promoter and using adenovirus to transfer the gene into cancer cells. The
chosen promoter is designed to be induced by agents already used in treating
cancer cells, such as radiation or cisplatin.
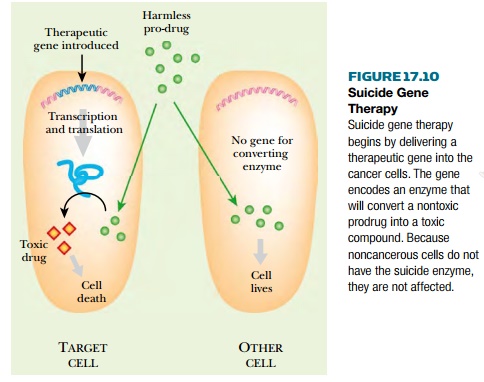
The suicide strategy is
actually a hybrid of anticancer drug therapy with gene transfer therapy.
A harmless compound, or prodrug,
is chosen that can be converted to a toxic anticancer drug by a specific
enzyme. If the enzyme is present, the cell expressing it will commit suicide
when the prodrug is available (Fig. 17.10). Consequently, an enzyme that is not
present in normal human cells must be chosen for this approach. The gene
encoding the suicide enzyme must be delivered to the target cancer cells,
usually by a viral vector or in liposomes. If the enzyme is successfully
expressed in the cancer tissue, then the toxic drug will be generated inside
the cancer cells. Thus the prodrug can be administered to the patient by normal
means but is specifically lethal for the cancer cells.
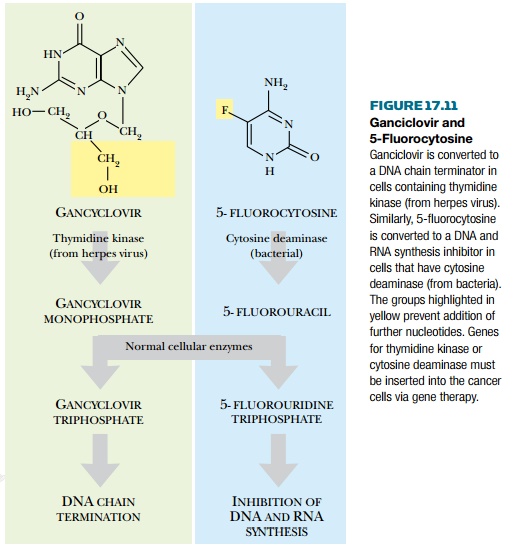
In practice two major suicide
enzyme/prodrug combinations have been used. Gene therapy has been used to
deliver the enzyme thymidine kinase,
originally from herpes virus, to cancer cells. The nontoxic prodrug, the
nucleoside analog ganciclovir, is
converted to its monophosphate by thymidine kinase (hence its clinical use in
treating herpes virus infections). Because only the cancer cells have thymidine
kinase, all the noncancerous cells are unaffected. Normal cellular enzymes then
convert the monophosphate to ganciclovir triphosphate (GCV-TP). This acts as a DNA chain terminator (Fig. 17.11). DNA
polymerase incorporates GCV-TP into growing strands of DNA. However, lack of a
3′-OH group prevents further
elongation of the nucleic acid strand. DNA synthesis is thus inhibited and the
cell is killed. A similar scheme involves the conversion of 5-fluorocytosine to
5-fluorouracil by cytosine deaminase,
originally from bacteria. Again, cellular enzymes finish the job by making the
phosphorylated nucleoside that actually inhibits DNA and RNA synthesis.
A more indirect approach
relies on the body’s natural defenses. Our immune systems are effective at
killing cancers, provided they identify them while still small. To survive, a
cancer has to somehow evade the body’s immune surveillance. In this approach,
gene therapy inserts a gene that attracts the attention of the immune system to
the tumor cells. For example, the HLA (= MHC) proteins are exposed on the
surfaces of mammalian cells where they act in cell recognition. Different
individuals have different combinations of HLA
genes, which act as molecular identity tags so that cells of the body are
recognized as “self.” If HLA genes that are not originally present in that
particular individual are inserted into the cancer cells, the tumor appears
alien, and the immune system will now mount an assault.
A related approach is to use cytokines. These are short proteins
that attract immune cells and stimulate their division and development. The
genes for several cytokines of the interleukin family (especially IL2, IL4, and
IL12) have been used to provoke immune attacks on cancer cells.
Related Topics