Chapter: Pharmaceutical Drug Analysis: Refractometry
Refractometry: Theory
THEORY
Lorentz and Lorentz in 1880, introduced the terminology specific refraction or refractivity which may be expressed as
:
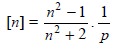 .............................(3)
.............................(3)
where, n = Refractive index,
p = Density of the substance*
Hence, the specific refraction (Eq. 3) is considered to
be a more useful property and is characteristic of the substance, being
absolutely independent of temperature.
Molar Refractivity :
Later on, a still more useful
property termed as the molar refraction (or refrac-tivity) was introduced which
could be expressed as follows :
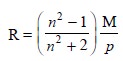 .............................................(4)
.............................................(4)
where, R = Molar refraction,
P = Density of the substance, and
M = Molecular weight.
Interestingly, both specific refraction [n] and molar refraction (R), being
temperature independent, should have the same values for a given substance
either in the solid, liquid or gaseous state, provided the molecular structure
is unchanged.
Unit of Molar Refraction :
As the refractive index is a
dimensionless quantity, the units of molar
refraction are simply those of molar volume, M/p i.e., cm3. mol–1.
The molar refractivity is more or less an additive
property.
Atomic Refractivities :
Atomic refractivities may be
attributed by virtue of :
(a) Structural
features e.g., double bond, triple
bond or nature of ring structure (3-member/4-member rings), and
(b) Individual
atoms, e.g., H, C, Cl, Br, I and O.
However, ‘O’ contributes different values for differ-ent groups, for instance :
hydroxyl (—OH), carbonyl (—CO—) and ethereal (—O—) moieties.
A few representative atomic refractivities and bond
contributions are given in Table 18.1 below :
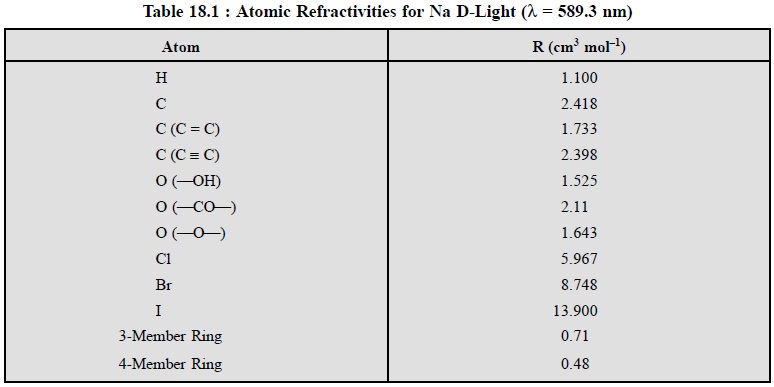
Based on the atomic refractivities given in Table 18.1,
it may be possible to calculate the molar refractivities of various
pharmaceutical substances theoretically and compare the same with values found
experimentally. A few typical examples are cited below :
(a) Acetone, CH3COCH3 [or C3H6O]
:
RC3H6O = 3 RC
+ 6RH + RO
=3 × 2.418 + 6 × 1.100 + 2.211
=7.254 + 6.600 + 2.11 =
15.964
Calculated : RC 3 H 6O = 15.964 cm3 mol–1
Experimental : RC 3 H 6O = 15.985 cm3 mol–1
(b) Methyl Alcohol, CH3OH [or CH4O]
:
RCH 3OH = RC + 4RH + RO
= 2.418 + 4 × 1.100 + 1.525
= 2.418 + 4.400 + 1.525
= 8.343
Calculated : RCH 4O = 8.343 cm3 mol–1
Experimental : RCH 4O = 8.296 cm3 mol–1
(c) Chloroform CHCl3 :
CHCl3 = RC + RH
+ 3RCl
= 2.418 + 1.100 + 3 × 5.967
= 2.418 + 1.100 + 17.901
= 21.419
Calculated : RCHCl3 = 21.419 cm3
mol–1
Experimental : RCHCl3 = 21.393 cm3
mol–1.
Related Topics