Chapter: Pharmaceutical Drug Analysis: Refractometry
Applications of Refractivity
APPLICATIONS OF REFRACTIVITY
The various applications of refractivity are enumerated
below :
(a) It is
feasible to determine the molar refractivities of different substances
experimentally and subsequently comparing their values with theoretical ones,
(b) Based on
the fact that molar refractivity is an additive property, it may be utilized to
determine the refractivities of homogeneous mixtures (as solutions).
Thus, the molar refraction of a solution having two components
(viz., the solute and the solvent) is
given by the expression :
R1, 2 = N1R1
+ N2R2 ...(i)
where, N1 = Mole fraction of the solute,
N2 = Mole fraction of the solvent,
R1 = Molar refractivity of the solute, and
R2 = Molar refractivity of the solvent.
Evidently, from Eq. (i),
it is quite possible to determine the molar refractivity of an unknown solute R1
provided we know the mole fraction N1 and N2 and the
refractivities of the solute R2 and the homogeneous solution R1,
2.
Besides, the concentration of the solute in the solution
may be determined by employing the follow-ing expression, provided the
refractivities of the solute, the solvent and the solution are known :
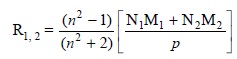 ..........................(ii)
..........................(ii)
(c) Determination of Critical Micelle
Concentration (CMC)
In general, substances that form micelles in water offer
two distinct regions in their molecules : first, the hydrophobic entity (caused
due to the hydrocarbon chain), and secondly, the hydrophilic entity (caused due
to the polar group). It has been observed that a number of monomers usually
hold all the hydrocarbon chains together specifically in the centre of the
micelle which are ultimately responsible for minimising the free energy of the
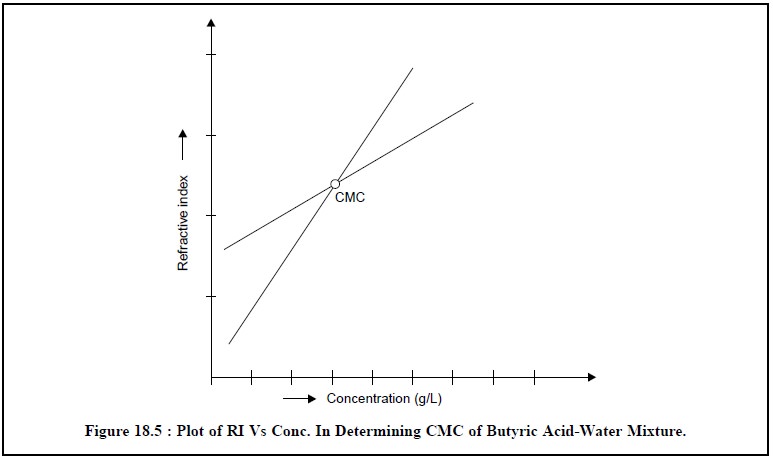
system. Thus, the particular concentration at which the micelles are first
observed is termed as the critical
micelle concentration (CMC). Interestingly, the physical characteristics of
the substances forming micelles afford sharp changes at the CMC. Therefore, a
plot of refractive index (RI) Vs concentration (g/L) must depict a visible
change in slope at the CMC.
Example :
Determination of critical micelle concentration (CMC) of Butyric Acid by refractometry :
Butryic acid (CH3CH2CH2COOH)
is found to form micelles in an aqueous medium in a concentra-tion range fairly
suitable for measurements with the Abbe’ refractometer. Materials Required : Butyric acid solution (25%, w/v in DW) : 200
ml ; volumetric flasks (50 ml) : 6 ;
Procedure :
Prepare precisely 2.5, 5.0,
7.5, 10.5, 15.0 and 20.0% solutions of butyric acid in water by measuring suitable volumes (from a
stock solution of 25% w/v) with the help of a burette into six 50 ml volumetric
flasks, and finally making up the volume with DW. Using Abbe’ refractometer
measure the refractive indices of all the above six solutions besides the stock
solution (25%) at 25°C. Measure also the refractive index of DW.
Results :
Plot a graph having the
various concentrations of butyric acid along the abscissa and the refractive indices along the ordinate,
whereby two straight lines are obtained intersecting at the CMC as shown in
Figure 18.5 below :
Related Topics