Chapter: Medical Physiology: Nervous Regulation of the Circulation, and Rapid Control of Arterial Pressure
Reflex Mechanisms for Maintaining Normal Arterial Pressure
Reflex Mechanisms for Maintaining Normal Arterial Pressure
Aside from the exercise and stress functions of the autonomic nervous system to increase arterial pressure, there are multiple subconscious special nervous control mechanisms that operate all the time to maintain the arterial pressure at or near normal. Almost all of these are negative feedback reflex mechanisms, which we explain in the following sections.
The Baroreceptor Arterial Pressure Control System—Baroreceptor Reflexes
By far the best known of the nervous mechanisms for arterial pressure control is the baroreceptor reflex. Basically, this reflex is initiated by stretch receptors, called either baroreceptors or pressoreceptors, located at specific points in the walls of several large systemic arteries. A rise in arterial pressure stretches the baroreceptors and causes them to transmit signals into the central nervous system. “Feedback” signals are then sent back through the autonomic nervous system to the circulation to reduce arterial pressure down-ward toward the normal level.
Physiologic Anatomy of the Baroreceptors and Their Innerva- tion. Baroreceptors are spray-type nerve endings thatlie in the walls of the arteries; they are stimulated when stretched. A few baroreceptors are located in the wall of almost every large artery of the thoracic and neck regions; but, as shown in Figure 18–5, baroreceptors are extremely abundant in (1) the wall of each inter-nal carotid artery slightly above the carotid bifurca-tion, an area known as the carotid sinus, and (2) the wall of the aortic arch.
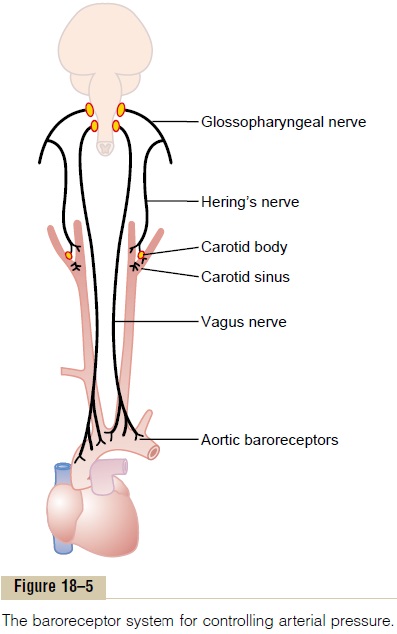
Figure 18–5 shows that signals from the “carotid baroreceptors” are transmitted through very small Hering’s nerves to theglossopharyngeal nerves inthe high neck, and then to the tractus solitarius in the medullary area of the brain stem. Signals from the “aortic baroreceptors” in the arch of the aorta are transmitted through the vagus nerves also to the same tractus solitarius of the medulla.
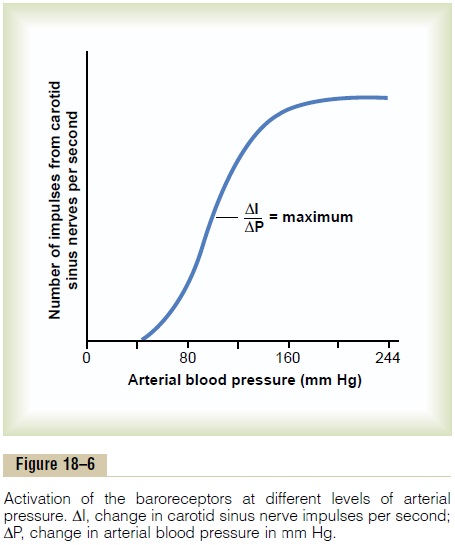
Response of the Baroreceptors to Pressure. Figure 18–6shows the effect of different arterial pressure levels on the rate of impulse transmission in a Hering’s carotid sinus nerve. Note that the carotid sinus baroreceptors are not stimulated at all by pressures between 0 and 50 to 60 mm Hg, but above these levels, they respond progressively more rapidly and reach a maximum at about 180 mm Hg. The responses of the aortic barore-ceptors are similar to those of the carotid receptors except that they operate, in general, at pressure levels about 30 mm Hg higher.
Note especially that in the normal operating range of arterial pressure, around 100 mm Hg, even a slight change in pressure causes a strong change in the baroreflex signal to readjust arterial pressure back toward normal. Thus, the baroreceptor feedback mechanism functions most effectively in the pressure range where it is most needed.
The baroreceptors respond extremely rapidly to changes in arterial pressure; in fact, the rate of impulse firing increases in the fraction of a second during each systole and decreases again during diastole. Further-more, the baroreceptors respond much more to arapidly changing pressure than to a stationary pres-sure. That is, if the mean arterial pressure is 150 mm Hg but at that moment is rising rapidly, the rate of impulse transmission may be as much as twice that when the pressure is stationary at 150 mm Hg.
Circulatory Reflex Initiated by the Baroreceptors. After thebaroreceptor signals have entered the tractus solitar-ius of the medulla, secondary signals inhibit the vaso-constrictor center of the medulla and excite the vagal parasympathetic center. The net effects are (1) vasodi-lation of the veins and arterioles throughout theperipheral circulatory system and (2) decreased heartrate and strength of heart contraction. Therefore, exci-tation of the baroreceptors by high pressure in the arteries reflexly causes the arterial pressure to decrease because of both a decrease in peripheral resistance and a decrease in cardiac output. Conversely, low pres-sure has opposite effects, reflexly causing the pressure to rise back toward normal.
Figure 18–7 shows a typical reflex change in arterial pressure caused by occluding the two common carotid arteries. This reduces the carotid sinus pressure; as a result, the baroreceptors become inactive and lose their inhibitory effect on the vasomotor center. The vasomotor center then becomes much more active than usual, causing the aortic arterial pressure to rise and remain elevated during the 10 minutes that the
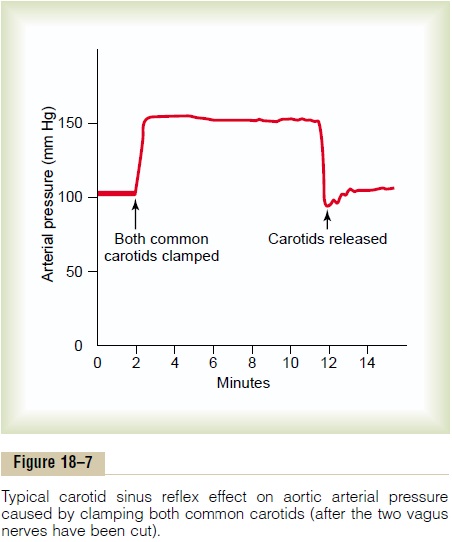
carotids are occluded. Removal of the occlusion allows the pressure in the carotid sinuses to rise, and the carotid sinus reflex now causes the aortic pressure to fall immediately to slightly below normal as a momen-tary overcompensation and then return to normal in another minute.
Function of the Baroreceptors During Changes in Body Posture. The ability of the baroreceptors to maintainrelatively constant arterial pressure in the upper body is important when a person stands up after having been lying down. Immediately on standing, the arterial pressure in the head and upper part of the body tends to fall, and marked reduction of this pressure could cause loss of consciousness. However, the falling pres-sure at the baroreceptors elicits an immediate reflex, resulting in strong sympathetic discharge throughout the body. This minimizes the decrease in pressure in the head and upper body.
Pressure “Buffer” Function of the Baroreceptor Control System. Because the baroreceptor systemopposes either increases or decreases in arterial pres-sure, it is called a pressure buffer system, and the nerves from the baroreceptors are called buffer nerves.
Figure 18–8 shows the importance of this buffer function of the baroreceptors. The upper record in this figure shows an arterial pressure recording for 2 hours from a normal dog, and the lower record shows an arterial pressure recording from a dog whose barore-ceptor nerves from both the carotid sinuses and the aorta had been removed. Note the extreme variability of pressure in the denervated dog caused by simple events of the day, such as lying down, standing, excite-ment, eating, defecation, and noises.
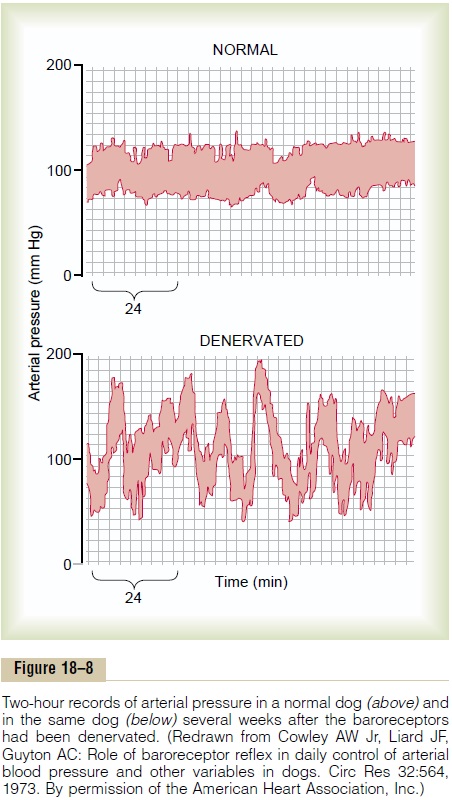
Figure 18–9 shows the frequency distributions of the mean arterial pressures recorded for a 24-hour day in both the normal dog and the denervated dog. Note that when the baroreceptors were functioning nor-mally the mean arterial pressure remained throughout the day within a narrow range between 85 and 115 mm Hg—indeed, during most of the day at almost exactly 100 mm Hg. Conversely, after denervation of the baroreceptors, the frequency distribution curve became the broad, low curve of the figure, showing that the pressure range increased 2.5-fold, frequently falling to as low as 50 mm Hg or rising to over 160 mm Hg. Thus, one can see the extreme variability of pres-sure in the absence of the arterial baroreceptor system.
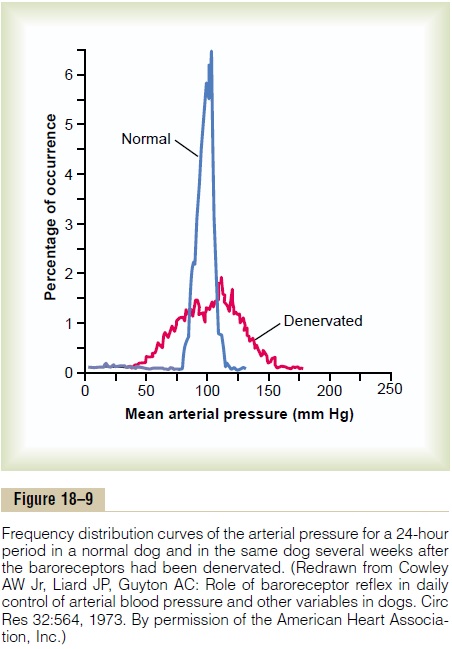
In summary, a primary purpose of the arterial baroreceptor system is to reduce the minute by minute variation in arterial pressure to about one third that which would occur were the baroreceptor system not present.
Are the Baroreceptors Important in Long-Term Regulation of Arterial Pressure? Although the arterial baroreceptorsprovide powerful moment-to-moment control of arterial pressure, their importance in long-term blood pressure regulation has been controversial.
One reason that the baroreceptors have been considered by some physiologists to be relatively unimportant in chronic regulation of arterial pressure chronically is that they tend to reset in 1 to 2 days to the pressure level to which they are exposed. That is, if the arterial pressure rises from the normal value of 100 mm Hg to 160 mm Hg, a very high rate of baroreceptor impulses are at first transmitted. During the next few minutes, the rate of firing diminishes considerably; then it diminishes much more slowly during the next 1 to 2 days, at the end of which time the rate of firing will have returned to nearly normal despite the fact that the mean arterial pressure still remains at 160 mm Hg. Conversely, when the arterial pressure falls to a very low level, the baroreceptors at first transmit no impulses, but gradually, over 1 to 2 days, the rate of baroreceptor firing returns toward the control level.
This “resetting” of the baroreceptors may attenuate their potency as a control system for correcting dis-turbances that tend to change arterial pressure for longer than a few days at a time. Experimental studies, however, have suggested that the baroreceptors do not completely reset and may therefore contribute to long-term blood pressure regulation, especially by influencing sympathetic nerve activity of the kidneys.
For example, with prolonged increases in arterial pres-sure, the baroreceptor reflexes may mediate decreases in renal sympathetic nerve activity that promote increased excretion of sodium and water by the kidneys. This, in turn, causes a gradual decrease in blood volume, which helps to restore arterial pressure toward normal. Thus, long-term regulation of mean arterial pressure by the baroreceptors requires interaction with additional systems, principally the renal–body fluid–pressure control system (along with its associated nervous and hormonal mechanisms).
Control of Arterial Pressure by the Carotid and Aortic Chemoreceptors—Effect of Oxygen Lack on Arterial Pressure.
Closely associated with the baroreceptor pressure control system is a chemoreceptor reflex that operates in much the same way as the baroreceptor reflex except that chemoreceptors, instead of stretch receptors, initi-ate the response.
The chemoreceptors are chemosensitive cells sensi-tive to oxygen lack, carbon dioxide excess, and hydro-gen ion excess. They are located in several small chemoreceptor organs about 2 millimeters in size (two carotid bodies, one of which lies in the bifurcation ofeach common carotid artery, and usually one to three aortic bodies adjacent to the aorta). The chemo-receptors excite nerve fibers that, along with the baroreceptor fibers, pass through Hering’s nerves and the vagus nerves into the vasomotor center of the brain stem.
Each carotid or aortic body is supplied with an abundant blood flow through a small nutrient artery, so that the chemoreceptors are always in close contact with arterial blood. Whenever the arterial pressure falls below a critical level, the chemoreceptors become stim-ulated because diminished blood flow causes decreased oxygen as well as excess buildup of carbon dioxide and hydrogen ions that are not removed by the slowly flowing blood.
The signals transmitted from the chemoreceptors excite the vasomotor center, and this elevates the arterialpressure back toward normal. However, this chemo-receptor reflex is not a powerful arterial pressure con-troller until the arterial pressure falls below 80 mm Hg. Therefore, it is at the lower pressures that this reflex becomes important to help prevent still further fall in pressure.
The chemoreceptors are discussed in much more detail in relation to respiratory control, in which they play a far more important role than in pressure control.
Atrial and Pulmonary Artery Reflexes That Help Regulate Arterial Pressure and Other Circulatory Factors. Both the atria andthe pulmonary arteries have in their walls stretch recep-tors called low-pressure receptors. They are similar to the baroreceptor stretch receptors of the large systemic arteries.These low-pressure receptors play an important role, especially in minimizing arterial pressure changes in response to changes in blood volume. To give an example, if 300 milliliters of blood suddenly are infused into a dog with allreceptors intact, the arterial pressure rises only about 15 mm Hg. With the arterial barorecep-tors denervated, the pressure rises about 40 mm Hg. Ifthe low-pressure receptors also are denervated, the pres-sure rises about 100 mm Hg.
Thus, one can see that even though the low-pressure receptors in the pulmonary artery and in the atria cannot detect the systemic arterial pressure, they do detect simultaneous increases in pressure in the low-pressure areas of the circulation caused by increase in volume, and they elicit reflexes parallel to the barore-ceptor reflexes to make the total reflex system more potent for control of arterial pressure.
Atrial Reflexes That Activate the Kidneys—The “Volume Reflex.”
Stretch of the atria also causes significant reflex dilation of the afferent arterioles in the kidneys. And still other signals are transmitted simultaneously from the atria to the hypothalamus to decrease secretion of antidiuretic hormone. The decreased afferent arteriolar resistance in the kidneys causes the glomerular capillary pressure to rise, with resultant increase in filtration of fluid into the kidney tubules. The diminution of antidi-uretic hormone diminishes the reabsorption of water from the tubules. Combination of these two effects— increase in glomerular filtration and decrease in reab-sorption of the fluid—increases fluid loss by the kidneys and reduces an increased blood volume back toward normal.
All these mechanisms that tend to return the blood volume back toward normal after a volume overload act indirectly as pressure controllers as well as blood volume controllers because excess volume drives the heart to greater cardiac output and leads, therefore, to greater arterial pressure. This volume reflex mechanism is discussed again, along with other mech-anisms of blood volume control.
Atrial Reflex Control of Heart Rate (the Bainbridge Reflex). Anincrease in atrial pressure also causes an increase in heart rate, sometimes increasing the heart rate as much as 75 per cent. A small part of this increase is caused by a direct effect of the increased atrial volume to stretch the sinus node: it was pointed out that such direct stretch can increase the heart rate as much as 15 per cent. An additional 40 to 60 per cent increase in rate is caused by a nervous reflex called the Bain-bridge reflex. The stretch receptors of the atria that elicitthe Bainbridge reflex transmit their afferent signals through the vagus nerves to the medulla of the brain. Then efferent signals are transmitted back through vagal and sympathetic nerves to increase heart rate and strength of heart contraction. Thus, this reflex helps prevent damming of blood in the veins, atria, and pul-monary circulation.
Related Topics